
Second Academic Year for Medication Safety Scholars Program
March 22, 2024
Really excited to share the news that we will be beginning the second academic year for the Emily Jerry Foundation‘s Medication Safety Scholars Program! This comprehensive distance education and virtual engagement program was developed and successfully implemented over the… Read More



Connecticut Scorecard
Grading Scale:
A – 85-100%, B – 70-84.9%, C – 55-69.9%, D – 40-54.9%, F – 0-39.9%
Grading Categories & Criteria
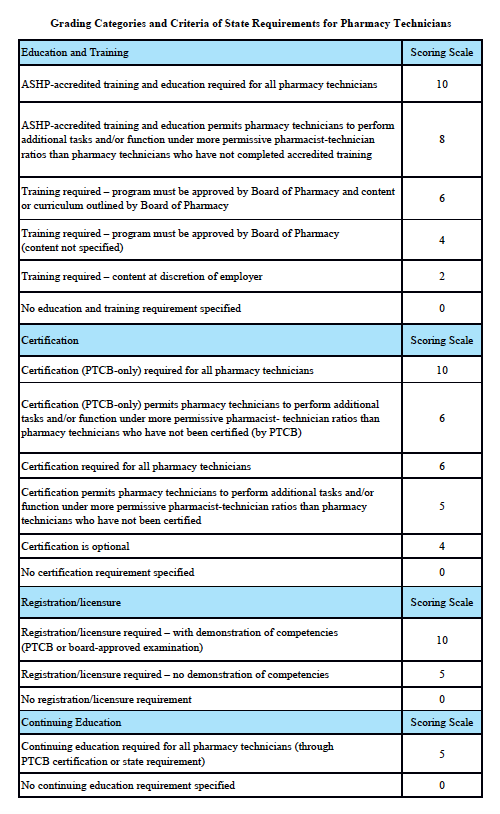
Connecticut Law
I. Laws
Sec. 20-571. (Formerly Sec. 20-184a). Definitions.
Sec. 20-598a. Registration and certification of pharmacy technicians.
Sec. 20-613. (Formerly Sec. 21a-308). Dispensing of drug or legend device pursuant to prescription only; exceptions. Emergency dispensing of drug or device in care-giving, correctional or juvenile training institutions; regulations. Pharmacy technicians. Prescribing practitioner authorized to dispense own prescription, when.
Sec. 20-607. (Formerly Sec. 20-173). Certificate of license, temporary permit or registration to be available for inspectionSec
Sec. 20-571. (Formerly Sec. 20-184a). Definitions.
(20) “Pharmacy technician” means an individual who is registered with the department and qualified in accordance with section 20-598a;
Sec. 20-598a. Registration and certification of pharmacy technicians.
(a) No person shall act as a pharmacy technician unless registered with, or certified with, the department.
(b) The department shall, upon authorization of the commission, register as a pharmacy technician any person who presents evidence satisfactory to the department that such person is qualified to perform, under the direct supervision of a pharmacist, routine functions in the dispensing of drugs that do not require the use of professional judgment. The qualifications for registration as a pharmacy technician under this section shall be in accordance with
(1) the standards of an institutional pharmacy, a care-giving institution or a correctional or juvenile training institution, in the case of employment in any such pharmacy or institution, or
(2) the standards established by regulation adopted by the commissioner in accordance with chapter 54, in the case of employment in a pharmacy. As used in this subsection, “direct supervision” means a supervising pharmacist
(A) is physically present in the area or location where the pharmacy technician is performing routine drug dispensing functions, and
(B) conducts in-process and final checks on the pharmacy technician’s performance. (c) The department shall, upon authorization of the commission, certify as a pharmacy technician any person who meets the requirements for registration as a pharmacy technician, pursuant to subsection (b) of this section, and who holds a certification from the Pharmacy Technician Certification Board or any other equivalent pharmacy technician certification program approved by the department.
(d) The fee required by section 20-601 shall accompany an application for registration under this section. A registration as a pharmacy technician shall be valid for one year and may be renewed upon application and payment of the fee required by section 20-601. (P.A. 98-31, S. 1: P.A. 99-175, S. 27; P.A. 04-208, S. 2.) History: P.A. 99-175 made technical changes in Subsecs. (a) and (b); P.A. 04-208 amended Subsec. (a) by adding “or certified with”, added new Subsec. (c) providing for the certification of pharmacy technicians, and relettered existing Subsec. (c) as Subsec. (d), effective June 3, 2004.
. 20-607. (Formerly Sec. 20-173). Certificate of license, temporary permit or registration to be available for inspection.
Each person practicing as a pharmacist, pharmacy intern or pharmacy technician shall at all times have available for inspection by an inspector of the department a current certificate of license or temporary permit to practice pharmacy or a current registration to act as a pharmacy intern or pharmacy technician.
II. Regulations
Sec. 20-576-32. Pharmacy technicians. Definitions
Sec. 20-576-37. Training and registration
Sec. 20-576-60. Definitions
Sec. 20-576-32. Pharmacy technicians. Definitions
• The definitions in section 20-571 of the Connecticut General Statutes and this section shall apply to sections 20-576-33 to 20-576-39 inclusive, of the Regulations of Connecticut State Agencies. The term pharmacy technician does not include:
• persons working in an institutional pharmacy who are not engaged in the compounding and dispensing of medications, such as stock clerks and clerical personnel; and
(2) persons working in a pharmacy who are not engaged in the compounding and dispensing of medications, such as stock clerks, cashiers, clerical personnel and data entry personnel performing routine functions such as entering and retrieving basic information not directly related to dispensing as defined in subdivision (9) of section 20-571 of the Connecticut General Statutes, getting prescription files and other manual records from storage, generating computer records such as refill logs and inventories of dispensing for the signature or initials of the pharmacist, handling or delivering completed prescriptions to the patient or the patient’s agent, and ringing up or receiving sales. Data entry of demographic and insurance information shall not be considered to be directly related to dispensing.
(b) “Supervising pharmacist” means a pharmacist who supervises pharmacy technicians; who is fully aware of and responsible for all activities pertinent to drug preparation, dispensing and distribution in which pharmacy technicians are engaged; and who conducts in-process and final checks on the performance of such pharmacy technicians.
(c) “Certified pharmacy technician” means a person who holds an active certification from the Pharmacy Technician Certification Board, or any other equivalent pharmacy technician certification approved by the Commission of Pharmacy.
(d) “Director of pharmacy” means the pharmacist designated by the facility administrator in a care-giving, correctional or juvenile training institution as being in direct charge of, and having overall responsibility for the operation and management of pharmacy services of that institution.
(e) “Inpatient pharmacy” means that area of an institutional pharmacy which is engaged in the manufacture, production, sale and distribution of drugs, devices and other pharmaceutical related materials used in the diagnosis and treatment of registered inpatients of a care-giving, correctional or juvenile training institution.
(f) “Satellite pharmacy” means an extension of an inpatient pharmacy which provides decentralized pharmaceutical care to persons in specific locations within a care-giving, correctional or juvenile training institution, including but not limited to specific patient care areas, nursing units, operating rooms and critical care units.
(g) “Outpatient pharmacy” means that area of an institutional pharmacy which provides pharmaceutical care to registered outpatients receiving treatment at a care-giving institution.
(Added effective January 11, 1999; Amended effective June 28, 2004.)
Sec. 20-576-37. Training and registration
(a) Pharmacy technicians shall complete initial training as determined by the pharmacist manager of each pharmacy. Such training shall include, but not be limited to, on-the-job and other related education and shall be commensurate with the tasks pharmacy technicians are to perform. This training shall be completed prior to the regular performance of such tasks. The pharmacy technician shall be registered with the department no more than thirty days after the start of such training.
(b) The pharmacist manager shall assure the continued competency of pharmacy technicians through continuing in-service training designed to supplement initial training.
(c) The pharmacist manager shall be responsible for maintaining a written record documenting the initial and continuing training of pharmacy technicians and it shall contain the following information:
(1) the name of the individual receiving the training;
(2) the date(s) of the training;
(3) a general description of the topics covered;
(4) the name of the person supervising the training; and
(5) the signature of the individual receiving the training and the pharmacist manager. When a change of pharmacist manager occurs, the new manager shall review the document and sign it, indicating that he understands its contents. This record shall be readily available for inspection and may be copied by the Commissioner of Consumer Protection or his authorized agents.
(Added effective January 11, 1999; Amended effective June 28, 2004.)
Sec. 20-576-60. Definitions
As used in sections 20-576-60 to 20-576-63, inclusive, of the Regulations of Connecticut State Agencies:
(1) “Agreement state” means any state that has entered into an agreement with the United States Nuclear Regulatory Commission or the Atomic Energy Commission under 42 U.S.C. § 2021;
(2) “Commission” means the Commission of Pharmacy;
(3) “Component” means any active or non-active ingredient of a drug product;
(4) “Department” means the Department of Consumer Protection;
(5) “Nuclear pharmacist” or “authorized nuclear pharmacist” means a pharmacist who holds a current pharmacist license issued by the commission, and who meets the following standards:
(A) has a current board certification as a nuclear pharmacist by the Board of Pharmaceutical Specialties; or
(B) is identified as an authorized nuclear pharmacist on a United States Nuclear Regulatory Commission or agreement state license that authorizes the use of radioactive material in the practice of nuclear pharmacy;
(6) “Nuclear pharmacy technician” means a person who:
(A) works under the direct supervision of a nuclear pharmacist;
(B) is currently registered as a pharmacy technician with the department; and
(C)
(i) has successfully completed a nuclear pharmacy technician training program provided by an accredited college program or an equivalent company sponsored program approved by the commission, or
(ii) is listed as an “Authorized User of Radioactive Materials” on the nuclear pharmacy’s United States Nuclear Regulatory Commission or agreement state license;
(7) “Nuclear pharmacy” means a pharmacy that provides radiopharmaceutical services and holds a Connecticut pharmacy license;
(8) “Practice of nuclear pharmacy” means a patient-oriented service that embodies the scientific knowledge and professional judgment required to improve and promote health through the assurance of the safe and efficacious use of radiopharmaceuticals and other drugs;
(9) “Quality assurance procedures” means all activities necessary to assure the quality of the process used to provide radiopharmaceutical services, including authentication of the product history, internal test assessment, and maintenance of all required records;
(10) “Quality control testing” means the performance of appropriate chemical, biological and physical tests on compounded and prepared radiopharmaceuticals and the interpretation of the resulting data to determine their suitability for use in humans and animals;
(11) “Radiopharmaceutical” means any drug that exhibits spontaneous disintegration of unstable nuclides with the emission of nuclear particles or photons and includes any non-radioactive reagent kit or radionuclide generator or eluates derived therefrom, which is intended to be used in preparation of any such substance. The term “radiopharmaceutical” includes, but is not limited to, positron-emission tomography agents, any biological product, including, but not limited to, blood formed element, antibody or peptide, that is labeled with a radionuclide or solely intended to be labeled with a radionuclide;
(12) “Radiopharmaceutical compounding” means the preparation, mixing, assembling, packaging, or labeling of a radiopharmaceutical that:
(A) is the result of a practitioner’s drug prescription order in the course of professional practice;
(B) is for the purpose of, or incident to, research, teaching, or chemical analysis and not for sale or dispensing;
(C) includes use of reagent kits and radiopharmaceuticals in anticipation of prescription drug orders based on routine, regularly observed prescribing patterns;
(D) is performed in accordance with the preparation instructions contained in the approved drug product labeling or other preparation directions as provided by the manufacturer;
(E) is performed in consideration of patient safety and efficacy, with validated procedures which deviate from the preparation instructions specified in the approved drug product labeling; or
(F) may utilize professional judgment, scientific knowledge, literature evidence and other reference materials according to current standards of practice as the basis for employing any deviations from the labeled preparation instructions or modifications to a radiopharmaceutical, if the final drug product, created as a result of any such deviations or modifications, is subjected to appropriate quality control testing necessary to confirm the presence of the desired radiopharmaceutical qualities;
(13)”Radiopharmaceutical services” means the procurement, storage, handling, compounding, preparation, labeling, quality control testing, dispensing, distribution, transfer, record keeping, and disposal of radiochemicals, radiopharmaceuticals and ancillary drugs, and also includes quality assurance procedures, radiological health activities, any consulting activities associated with the use of radiopharmaceuticals, health physics, and any other activities required for the provision of pharmaceutical care; and
(14) “Reagent kit” means a sterile and pyrogen-free reaction vial containing nonradioactive chemicals, including, but not limited to, complexing agent (ligand), reducing agent, stabilizer, or dispersing agent.
(Added effective November 30, 2006.)
References
Pharmacy Practice Act
http://www.cga.ct.gov/2011/pub/chap400j.htm
Regulations on the Practice of Pharmacy
http://www.ct.gov/dcp/lib/dcp/dcp_regulations/20-576_the_practice_of_pharmacy.pdf
The data contained in this 2012 Annual Scorecard are accurate as of December 2012 . Because statutes and regulations are continually revised, the data are subject to change. These data have been verified with the state board of pharmacy. This scorecard is updated on an annual basis in order to incorporate statutory and regulatory changes. A new scorecard will be issued in July 2013.
Scoring rationale for Education and Training:
In order to protect the public and help ensure patient safety, it is important that pharmacy technicians are properly educated and trained. The most rigorous training is accredited training. The sole entity empowered to accredit pharmacy-technician training programs is the American Society of Health-System Pharmacists (ASHP). Please note that this is “programmatic accreditation” – not “institutional accreditation.” It is the content of the training program – as measured against established standards – that is being evaluated and accredited. Accredited training is vital to protecting patient safety because it means that a pharmacy-technician training program has met established quality standards to provide assurance and confidence to the public. For more information, please see http://www.ashp.org/menu/Accreditation/TechnicianAccreditation.aspx.
Scoring rationale for Certification:
Certification is the process by which a nongovernmental agency or association grants recognition to an individual who has met certain predetermined qualifications specified by that agency or association. This is often determined by an examination process. Numerous organizations have recommended that the certification exam conducted by the Pharmacy Technician Certification Board (PTCB) should be recognized as the sole, nationally-accredited certification exam for pharmacy technician certification – including the National Association of Boards of Pharmacy (NABP), the Texas State Board of Pharmacy (TSBP), and the U.S. Department of Veterans Affairs (VA). In a recent report, NABP recommended that states be encouraged to “recognize certification by the Pharmacy Technician Certification Board (PTCB).” Moreover, NABP performed a psychometric audit of the PTCB’s pharmacy technician certification examination (PTCE) in 2001 and determined that the PTCE is psychometrically sound, defensible, and valid. In May 2010, the TSBP awarded the PTCB with the Pharmacy Technician Certification Provider contract in Texas. PTCB was selected for the contract after a rigorous bidding and evaluation process that included formal reviews and evaluations from three independent psychometricians. TSBP confidently recognizes PTCB as the single provider of certification examinations for pharmacy technicians. In addition, in June 2010, the VA began requiring PTCB certification for VA pharmacy technicians employed at grade GS-6 and above.
Scoring rationale for Registration/Licensure:
Registration/licensure is the process by which the state maintains a list of all pharmacy technicians in the state and grants permission for an individual to work as a pharmacy technician in the state based on the applicant’s completion of all pre-requisites to registration/licensure – such as required training and certification.
Scoring rationale for Continuing Education:
Continuing education enables pharmacy technicians to fulfill their professional responsibility to maintain competence and up-to-date knowledge and skills in an environment of technological advances and increasingly complex, new medications and therapies.
Our Mission
The Emily Jerry Foundation is determined to help make our nation’s, world renowned, medical facilities safer for everyone, beginning with our babies and children. We are accomplishing this very important objective by focusing on increasing public awareness of key patient safety related issues and identifying technology and best practices that are proven to minimize the “human error” component of medicine. Through our ongoing efforts The Emily Jerry Foundation is working hard to save lives every day.
Recent Posts
Archives