
Keynote at Mount Sinai Health System’s Medication Safety Together Summit
November 24, 2025
By ejfadmin
I was truly honored to represent the Emily Jerry Foundation last week at Mount Sinai Health System, where I had the privilege of delivering the keynote address to kick off their Medication Safety Together – An Interdisciplinary Summit… Read More



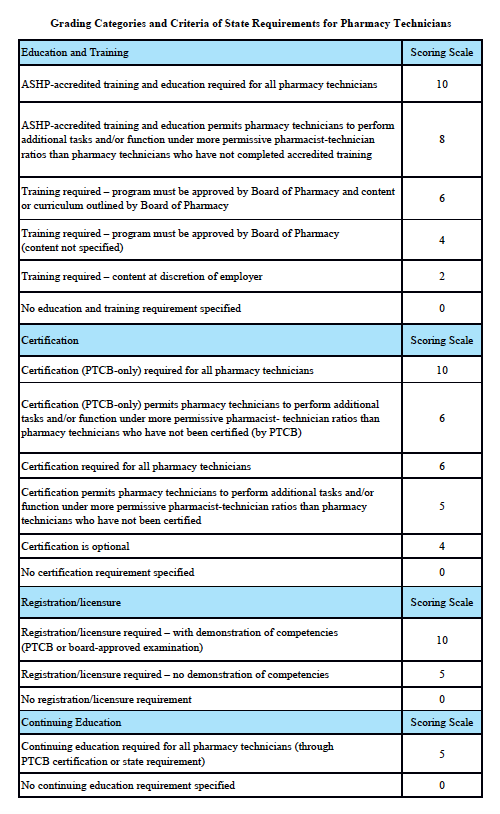
Michigan Scorecard
Grading Scale:
A – 85-100%, B – 70-84.9%, C – 55-69.9%, D – 40-54.9%, F – 0-39.9%
Grading Categories & Criteria
Michigan Law
https://www.legislature.mi.gov/Laws/MCL?objectName=mcl-368-1978-15-177
333.17711 Practice of pharmacy or pharmacy technician; license or authorization required; use of words, titles, or letters.
Sec. 17711.
(1) An individual shall not engage in the practice of pharmacy unless licensed or otherwise authorized by this article. Beginning October 1, 2015, an individual shall not serve as a pharmacy technician unless licensed or otherwise authorized by this article.
(2) The following words, titles, or letters or a combination of words, titles, or letters, with or without qualifying words or phrases, are restricted in use only to those persons authorized under this part to use the terms and in a way prescribed in this part: “”pharmacy””, “”pharmacist””, “”Pharm.D””, “”doctor of pharmacy””, “”pharmacy intern””, “”pharmacy technician””, “”licensed pharmacy technician””, “”certified pharmacy technician””, “”CPhT””, “”apothecary””, “”dispensary””, “”drugstore””, “”druggist””, “”medicine store””, “”prescriptions””, and “”r.ph.””.
History: 1978, Act 368, Eff. Sept. 30, 1978 ;– Am. 2006, Act 390, Imd. Eff. Sept. 27, 2006 ;– Am. 2014, Act 285, Eff. Dec. 22, 2014 ;– Am. 2014, Act 413, Eff. Mar. 30, 2015 ;– Am. 2015, Act 91, Imd. Eff. June 25, 2015
Popular Name: Act 368
333.17739 Pharmacy technician; functions; licensure.
Sec. 17739.
(1) An individual who performs any of the following functions is considered to be serving as a pharmacy technician and, except as otherwise provided in this part, is required to be licensed under this part as a pharmacy technician:
(a) Assisting in the dispensing process.
(b) Handling transfer of prescriptions, except controlled substances prescriptions.
(c) Compounding drugs.
(d) Preparing or mixing intravenous drugs for injection into a human patient.
(e) Contacting prescribers concerning prescription drug order clarification, which does not include drug regimen review or clinical or therapeutic interpretation.
(f) Receiving verbal orders for prescription drugs, except orders for controlled substances.
(g) Subject to section 16215, performing any other functions authorized under rules promulgated by the department in consultation with the board.
(2) A pharmacy or dispensing prescriber that utilizes the services of a pharmacy technician shall ensure that all of the following requirements, as applicable, are met:
(a) The pharmacy technician is licensed or otherwise authorized to serve as a pharmacy technician under this part.
(b) The pharmacy technician only performs the activities or functions that he or she is licensed or otherwise authorized to perform under this part or rules promulgated under this part.
(c) Except for a remote pharmacy or as otherwise provided by rule promulgated by the department in consultation with the board, the pharmacy technician only performs the activities or functions described in subdivision (b) under the supervision and personal charge of the pharmacist or dispensing prescriber.
History: Add. 2014, Act 285, Eff. Dec. 22, 2014 ;– Am. 2020, Act 4, Eff. Apr. 26, 2020
Popular Name: Act 368
333.17739a Pharmacy technician; licensure; requirements; exemption from certain requirements.
Sec. 17739a.
(1) Subject to subsection (2), the department may license an individual who meets all of the following requirements as a pharmacy technician under this part:
(a) Submits a completed application to the department on a form prescribed by the department.
(b) Except as otherwise provided in subsection (4), graduated from an accredited high school or comparable school or educational institution or passed the general educational development test or other graduate equivalency examination.
(c) Satisfies the requirements of section 16174.
(d) Except as otherwise provided in subsection (4), passes and submits proof to the department of passage of any of the following:
(i) The certified pharmacy technician examination given by the Pharmacy Technician Certification Board.
(ii) The certified pharmacy technician examination given by the National Healthcareer Association.
(iii) Any other nationally recognized and administered certification examination approved by the board.
(iv) An employer-based training program examination that is approved by the board and covers job descriptions, pharmacy security, commonly used medical abbreviations, routes of administration, product selection, final check by pharmacists, guidelines for the use of pharmacy technicians, pharmacy terminology, basic drug information, basic calculations, quality control procedures, state and federal laws and regulations regarding pharmacy technician duties, pharmacist duties, pharmacy intern duties, prescription or drug order processing procedures, drug record-keeping requirements, patient confidentiality, and pharmacy security and drug storage.
(2) An individual who is not a pharmacist, pharmacist intern, or pharmacy technician shall not perform any of the functions described in section 17739(1) for a pharmacy.
(3) A pharmacist shall not allow any individual employed or otherwise under the personal charge of the pharmacist to violate subsection (2). A person that owns, manages, operates, or conducts a pharmacy shall not allow any individual employed or otherwise under the control of that person to violate subsection (2).
(4) An individual who meets any of the following is not required to meet the requirements of subsection (1)(b) and (d) to be eligible for a license under subsection (1):
(a) As provided in section 16171(a), is a student in a pharmacy technician program approved by the board.
(b) Is applying for a temporary license under section 17739b.
(c) Is applying for a limited license under section 17739c.
History: Add. 2014, Act 285, Eff. Dec. 22, 2014 ;– Am. 2015, Act 133, Imd. Eff. Sept. 30, 2015
Popular Name: Act 368
333.17739b Pharmacy technician; temporary license.
Sec. 17739b.
(1) Subject to section 17739a(4), the department may issue a temporary license as a pharmacy technician to an individual who is preparing for the examination under section 17739a(1)(d). Notwithstanding section 16181, the term of a temporary license issued under this section expires 1 year after the date the temporary license is issued.
(2) An individual requesting a temporary license under this section shall submit a completed application, on a form prescribed by the department, to the department and pay the applicable fee under section 16333.
(3) An individual who holds a temporary license as a pharmacy technician issued under subsection (1) is subject to all of the requirements of this part, and rules promulgated by the department in consultation with the board, applicable to pharmacy technicians except the examination requirement under section 17739a(1)(d).
History: Add. 2014, Act 285, Eff. Dec. 22, 2014 ;– Am. 2015, Act 133, Imd. Eff. Sept. 30, 2015
Popular Name: Act 368
333.17739c Pharmacy technician; limited license.
Sec. 17739c.
(1) In addition to the requirement of section 16182 and subject to section 17739a(4), the department may issue a limited license as a pharmacy technician to an individual if all of the following are met:
(a) The individual was employed as a pharmacy technician by a pharmacy on December 22, 2014 and has been continuously employed by that pharmacy since that date.
(b) The individual submits a completed application to the department on a form prescribed by the department and meets the requirements of section 16174.
(c) The individual provides documentation of satisfactory employment as a pharmacy technician for a minimum of 1,000 hours during the 2-year period immediately preceding the date of his or her application under subdivision (b).
(d) The applicable fee under section 16333 is paid.
(2) Except as otherwise provided in subsection (5), an individual who holds a limited license under this section may only act as a pharmacy technician for the pharmacy described in subsection (1)(a) and only until 1 of the following occurs:
(a) He or she is no longer employed by that pharmacy to perform those functions.
(b) He or she performs any of those functions for another pharmacy.
(3) The term of a limited pharmacy technician license issued by the department under this section is the same as a pharmacy technician license issued by the department under section 17739a.
(4) An individual who holds a limited pharmacy technician license issued under this section is subject to all of the requirements of this part, and the rules promulgated by the department in consultation with the board, except the examination requirement under section 17739a(1)(d).
(5) An individual who is employed as a pharmacy technician by an employer that operates multiple licensed pharmacy locations may work as a limited license pharmacy technician at any of the employer’s licensed pharmacy locations in this state.
History: Add. 2014, Act 285, Eff. Dec. 22, 2014 ;– Am. 2015, Act 133, Imd. Eff. Sept. 30, 2015
Popular Name: Act 368
Michigan Administrative Code
https://ars.apps.lara.state.mi.us/AdminCode/DeptBureauAdminCode?Department=Licensing%20and%20Regulatory%20Affairs&Bureau=All
R 338.3651 Definitions.
Rule 1. (1) As used in these rules:
(a) “ASHP/ACPE” means the American Society of Health-System Pharmacists/Accreditation
Council for Pharmacy Education.
(b) “Board” means the Michigan Board of Pharmacy.
(c) “CCAPP” means the Canadian Council for Accreditation of Pharmacy Programs.
(d) “Code” means the public health code, 1978 PA 368, MCL 333.1101 to 333.25211.
(e) “Department” means the department of licensing and regulatory affairs.
(f) “NHA” means the National Healthcareer Association.
(g) “PTCB” means the Pharmacy Technician Certification Board.
(2) Unless otherwise defined in these rules, the terms defined in the code have the same
meaning when used in these rules.
History: 2016 AACS; 2021 AACS; 2023 MR 19, Eff. Oct. 2, 2023
R 338.3651a Pharmacy technician licensure; eligibility; examination.
Rule 1a. (1) An applicant for licensure by examination shall submit a completed application on a form provided by the department, together with the appropriate fee, unless the applicant is exempt from filing under any of the following exemptions pursuant to section 17739a(4) of the code, MCL 333.17739a:
(a) A student, while the student is enrolled in a pharmacy technician program approved by the board under R 338.3655.
(b) A licensee who holds a temporary pharmacy technician license under R 338.3652 and section 17739b of the code, MCL 333.17739b.
(c) A licensee who holds a limited pharmacy technician license under section 17739c of the code, MCL 333.17739c.
(2) In addition to meeting the requirements of R 338.7001 to R 338.7004, any other rule promulgated under the code, and section 16174 of the code, MCL 333.16174, an applicant shall comply with all of the following requirements:
(a) Have graduated from an accredited high school or comparable school or educational institution or passed the general educational development test or the graduate equivalency examination.
(b) Have passed, and provided proof to the department of passing, any of the following examinations:
(i) The certified pharmacy technician examination given by the PTCB or the NHA.
(ii) A nationally recognized and administered pharmacy technician certification examination that has been approved by the board under R 338.3654.
(iii) An employer-based training program examination that has been approved by the board under R 338.3654.
(c) An applicant shall submit proof of having completed the 1-time training in identifying victims of human trafficking as required in R 338.3659 and section 16148 of the code, MCL 333.16148.
(3) An applicant who is or has ever been licensed, registered, or certified in a health profession or specialty by another state, the United States military, the federal government, or another country, shall do both of the following:
(a) Disclose each license, registration, or certification on the application form.
(b) Satisfy the requirements of section 16174(2) of the code, MCL 333.16174, which includes verification from the issuing entity showing that disciplinary proceedings are not pending against
the applicant and sanctions are not in force at the time of application.
History: 2023 MR 19, Eff. Oct. 2, 2023.
R 338.3652 Temporary license.
Rule 2. (1) Subject to the limitations in section 16181 of the code, MCL 333.16181, and under section 17739b of the code, MCL 333.17739b, the department may issue a nonrenewable, temporary license to an applicant who is preparing for the proficiency examination and has completed all requirements for licensure as a pharmacy technician under R 338.3651a except passing the proficiency examination required under section 17739a(1)(d) of the code, MCL 333.17739a.
(2) An applicant applying for a pharmacy technician temporary license shall submit a completed application on a form provided by the department, together with the appropriate fee.
(3) The temporary license expires 1 year after the date the temporary license is issued.
History: 2021 AACS; 2023 MR 19, Eff. Oct. 2, 2023. ” “R 338.3653 Licensure by endorsement.
Rule 3. (1) An applicant who has never held a pharmacy technician license in this state, but who is licensed in another state, may apply for licensure by endorsement by submitting a completed application on a form provided by the department, together with the requisite fee.
(2) An applicant is presumed to meet the requirements of section 16186 of the code, MCL 333.16186, if they meet the requirements of R 338.7001 to R 338.7004, any other rule promulgated under the code, and section 16174 of the code, MCL 333.16174, as well as all of the following requirements:
(a) Has graduated from an accredited high school or comparable school or educational institution or passed the general educational development test or the graduate equivalency examination.
(b) Satisfies the requirements in section 16174(2) of the code, MCL 333.16174, which includes verification from the issuing entity showing that disciplinary proceedings are not pending against
the applicant and sanctions are not in force at the time of application.
(c) Holds a pharmacy technician license or registration by examination in another state that is active and in good standing.
(d) Submits proof that the applicant passed 1 of the approved examinations specified in R 338.3651a(2)(b).
(e) Submits proof of having completed the 1-time training in identifying victims of human trafficking as required in R 338.3659 and section 16148 of the code, MCL 333.16148.
(f) Discloses each license, registration, or certification in a health profession or specialty issued by another state, the United States military, the federal government, or another country on the
application form.
History: 2016 AACS; 2021 AACS; 2023 MR 19, Eff. Oct. 2, 2023. ” “R 338.3654 Examination requirements; board approval; approval process.
Rule 4. (1) Except for the PTCB and NHA examinations, a nationally recognized pharmacy technician proficiency certification examination and an employer-based training program proficiency examination must be approved by the board.
(2) An employer-based training program proficiency examination must be offered in association with a specific employer-based training program and cover the topics specified in section 17739a(1)(d)(iv) of the code, MCL 333.17739a.
(3) An entity that offers a nationally recognized pharmacy technician proficiency certification examination shall submit to the department a completed application on a form provided by the department with proof of current national accreditation in order to be approved by the board. If the examination is nationally accredited, after the department processes the application, it must be considered approved by the board. If national accreditation is lost, the examination will no longer be approved by the board.
(4) An entity that offers an employer-based training program proficiency examination shall submit to the department a completed application on a form provided by the department and a copy of the examination with the correct answers clearly identified for each question.
(5) An entity that offers an employer-based training program proficiency examination shall submit a modification to a proficiency examination during its approval term to the department on a form provided by the department pursuant to the requirements of this rule.
(6) Except for PTCB and NHA, a nationally recognized certification proficiency examination or employer-based training program proficiency examination approved by the board before July 1,
2022, shall submit an application consistent with this rule for approval by December 31, 2023, or the program will no longer be listed as a board-approved program.
(7) The board’s approval of an examination expires 5 years after the date of approval.
(8) One year after the effective date of this subrule, a board-approved program must include a proficiency examination grading procedure with the proficiency examination application, which will be reviewed by the board with the examination. ” “R 338.3655 Approved pharmacy technician programs.
Rule 5. (1) The following pharmacy technician programs are considered board-approved after a completed application on a form provided by the department along with proof of accreditation is submitted to and reviewed by the department:
(a) A pharmacy technician program including an employer-based training program that is accredited by the ASHP/ACPE Pharmacy Technician Accreditation Commission.
(b) A pharmacy technician program that is offered by an education program that is accredited by the ASHP/ACPE Pharmacy Technician Accreditation Commission or by an agency accredited by the United States Department of Education.
(2) If any of the following pharmacy technician programs do not meet the requirements in subrule (1) of this rule, the program may apply for board approval by submitting an application to the department on a form provided by the department, along with an attestation form that verifies compliance with the information required in subrule (3) of this rule:
(a) A comprehensive curriculum-based pharmacy technician education and training program conducted by a community college under the community college act of 1966, 1966 PA 331, MCL 389.1 to 389.195 or a school that is licensed under the proprietary schools act, 1943 PA 148, MCL 395.101 to 395.103.
(b) A pharmacy technician training program utilized by a pharmacy that includes training in the functions, specified in section 17739(1) of the code, MCL 333.17739, and R 338.3665, required to assist the pharmacist in the technical functions associated with the practice of
pharmacy.
(3) The contents of the training programs offered under subrule (2) of this rule must include all of the following:
(a) The duties and responsibilities of the pharmacy technician and a pharmacist, including the standards of patient confidentiality, and ethics governing pharmacy practice.
(b) The tasks and technical skills, policies, and procedures related to the pharmacy technician’s position pursuant to the duties specified in section 17739(1) of the code, MCL 333.17739, and R
338.3665.
(c) The pharmaceutical-medical terminology, abbreviations, and symbols commonly used in prescriptions and drug orders.
(d) The general storage, packaging, and labeling requirements of drugs, prescriptions, or drug orders.
(e) The arithmetic calculations required for the usual dosage determinations.
(f) The essential functions related to drug, purchasing, and inventory control.
(g) The recordkeeping functions associated with prescriptions or drug orders.
(4) The pharmacy technician program shall maintain a record of a student’s pharmacy technician training and education, specified in this rule, for 3 years after a student completes or leaves the program, whichever is earlier, that must include all of the following:
(a) The full name and date of birth of the pharmacy technician student.
(b) The starting date of the pharmacy technician program and date the student successfully completed the program.
(c) The program syllabus and activities performed in the program.
(5) A student shall complete a board-approved pharmacy technician program within 2 years of beginning the program in order to maintain the student’s exemption from licensure in subrule (6) of this rule, and R 338.3651a.
(6) A student in a board-approved pharmacy technician program is exempt from licensure while in the program.
(7) A student who is at least 16 years of age, in a board-approved pharmacy technician program, may participate in practical hands-on training in the pharmacy.
(8) A pharmacy technician program that was board approved before July 1, 2022, shall reapply and meet the requirements of this rule no later than 1 year after these rules are promulgated, or
the program will no longer be listed as a board-approved program. The board’s approval of a program expires 5 years after the date of approval. After 5 years, upon review by the department, a pharmacy technician program may be reapproved if it has maintained its accreditation.
(9) If the department determines that a board-approved program is not meeting the standards of the code or these rules, the department may send written notice to the program stating which
areas in the program are deficient. The program has 30 days to fix any deficiency and report back to the department. If the department determines that the deficiencies are not resolved, the board will evaluate the deficiencies and may withdraw approval.
(10) Withdrawal of board approval of a program for stated deficiencies that were not remediated does not make any bona fide student enrolled in the program, at the time of withdrawal of approval, ineligible to sit for an approved licensure examination.
History: 2016 AACS; 2021 AACS; 2023 MR 19, Eff. Oct. 2, 2023.” “R 338.3657 Relicensure requirements for pharmacy technicians.
Rule 7. (1) An applicant for relicensure whose pharmacy technician license has lapsed in this state under section 16201(3) or (4) of the code, MCL 333.16201, as applicable, may be relicensed by complying with the following requirements:
copy forward table located on page 5-7
https://ars.apps.lara.state.mi.us/AdminCode/DownloadAdminCodeFile?FileName=R%20338.3651%20to%20R%20338.3665.pdf
(2) If relicensure is granted and it is determined that a sanction has been imposed by another state, the United States military, the federal government, or another country, the disciplinary subcommittee may impose appropriate sanctions under section 16174(5) of the code, MCL 333.16174.
History: 2016 AACS; 2021 AACS; 2023 MR 19, Eff. Oct. 2, 2023. ” “R 338.3659 Training standards for identifying victims of human trafficking; requirements.
Rule 9. (1) Under section 16148 of the code, MCL 333.16148, the individual licensed or seeking licensure shall have completed training in identifying victims of human trafficking that
meets the following standards:
(a) Training content covers all of the following:
(i) Understanding the types and venues of human trafficking in the United States.
(ii) Identifying victims of human trafficking in healthcare settings.
(iii) Identifying the warning signs of human trafficking in healthcare settings for adults and minors.
(iv) Identifying resources for reporting the suspected victims of human trafficking.
(b) Acceptable providers or methods of training include any of the following:
(i) Training offered by a nationally recognized or state-recognized health-related organization.
(ii) Training offered by, or in conjunction with, a state or federal agency.
(iii) Training obtained in an educational program that has been approved by the board for initial licensure, or by a college or university.
(iv) Reading an article related to the identification of victims of human trafficking that meets the requirements of subdivision (a) of this subrule and is published in a peer-review journal, healthcare journal, or professional or scientific journal.
(c) Acceptable modalities of training include any of the following:
(i) Teleconference or webinar.
(ii) Online presentation.
(iii) Live presentation.
(iv) Printed or electronic media.
(2) The department may select and audit an individual and request documentation of proof of completion of training. If audited by the department, the individual shall provide an acceptable
proof of completion of training, including either of the following:
(a) Proof of completion certificate issued by the training provider that includes the date, provider name, name of training, and individual’s name.
(b) A self-certification statement by the individual. The certification statement must include the individual’s name and 1 of the following:
(i) For training completed under subrule (1)(b)(i) to (iii) of this rule, the date, training provider name, and name of training.
(ii) For training completed pursuant to subrule (1)(b)(iv) of this rule, the title of article, author, publication name of the peer-review journal, healthcare journal, or professional or scientific journal, and the date, volume, and issue of publication, as applicable.
History: 2016 AACS; 2021 AACS; 2023 MR 19, Eff. Oct. 2, 2023.” “R 338.3661 License renewals; continuing education requirements.
Rule 11. (1) This rule applies to applications for renewal of a pharmacy technician’s license and a special retired volunteer pharmacy technician’s license under sections 16184 and 16201 of the code, MCL 333.16184 and 333.16201. A licensee seeking renewal shall comply with all of the following:
(a) Submit to the department a completed application for renewal on a form provided by the department together with the requisite fee.
(b) Complete the training in identifying victims of human trafficking as required in R 338.3659.
(c) An applicant for license renewal, who has been licensed for the entire 2-year period preceding the end of the license cycle, shall during the 2 years immediately preceding the application for renewal complete not less than 20 hours of continuing education approved by the board under R 338.3662 as follows:
(i) An applicant for license renewal shall complete 1 hour in pharmacy ethics and jurisprudence, which may be completed in 1 or more courses.
(ii) An applicant for license renewal shall complete 1 hour in pain and symptom management in the practice of pharmacy that includes, but is not limited to, courses in the following subjects:
(A) Behavior management.
(B) Psychology of pain.
(C) Pharmacology.
(D) Behavior modification.
(E) Stress management.
(F) Clinical applications as they relate to professional practice.
(iii) An applicant for license renewal shall complete 1 hour in patient safety.
(iv) An applicant for license renewal shall earn no more than 12 hours of continuing education during a 24-hour period.
(v) Except for the 1-time training in human trafficking and the implicit bias training, which may be used to comply with both the training requirement and the continuing education requirement in the same renewal period, an applicant for license renewal may not earn continuing education credit for a program or activity that is identical to a program or activity an applicant has already earned credit for during that renewal period.
(vi) An applicant for license renewal shall earn at least 5 hours of continuing education in live, synchronous, courses or programs, in-person or virtual, that provide for the opportunity of direct interaction between faculty and participants, including, but not limited to, lectures, symposia, live teleconferences, and workshops. ACPE courses designated as live meet this requirement.
(2) Submission of an application for renewal constitutes the applicant’s certification of compliance with this rule. The licensee shall retain documentation of meeting the requirements of this rule for a period of 4 years after the date of applying for license renewal. Failure to comply with this rule is a violation of section 16221(h) of the code, MCL 333.16221.
(3) A request for a waiver under section 16205 of the code, MCL 333.16205, must be received by the department for the board’s consideration at least 30 days before the last regularly scheduled board meeting before the expiration date of the license. The public notice for the board meetings can be found here:
https://www.michigan.gov/lara/bureau-list/bpl/health/hp-lic-health-prof/pharmacy.
(4) Continuing education that is earned during the 60-day grace period may be included up to the date the application for renewal is filed.
History: 2016 AACS; 2021 AACS; 2023 MR 19, Eff. Oct. 2, 2023. ” “R 338.3662 Format of acceptable continuing education for licensees.
Rule 12. The board shall consider all of the following as acceptable continuing education:
copy forward table located on page 9
https://ars.apps.lara.state.mi.us/AdminCode/DownloadAdminCodeFile?FileName=R%20338.3651%20to%20R%20338.3665.pdf
R 338.3663 Continuing education courses and programs; standards for approval.
Rule 13. A continuing education course or program that is not pre-approved under R 338.3662(a) may be approved by the board pursuant to the standards in R 338.3043.
History: 2016 AACS; 2021 AACS; 2023 MR 19, Eff. Oct. 2, 2023.
R 338.3665 Performance of activities and functions; delegation.
Rule 15. In addition to performing the functions described in section 17739(1) of the code,
MCL 333.17739, a licensed pharmacy technician may also engage in the following tasks, under the delegation and supervision of a licensed pharmacist:
(a) Reconstitute non-sterile dosage forms consistent with approved labeling provided by the manufacturer of a commercially available product.
(b) Provide technology-assisted final product verification, which includes all the following:
(i) A properly trained pharmacy technician performing final product verification with the use of bar coding or another error prevention technology.
(ii) The licensed pharmacy technician providing final product verification is subject to all of the following requirements:
(A) The licensed pharmacy technician holds a current full or limited pharmacy technician
license in this state.
(B) Before performing final product verification the full or limited licensed pharmacy
technician meets 1 of the following:
(1) Has accrued not less than 1,000 hours of pharmacy technician work experience in the
same type of pharmacy practice site where the technology-assisted final product verification will be performed while the pharmacy technician holds a current full pharmacy technician license, a temporary license, a limited license, or is in training in this state.
(2) Has completed a final product verification training program that includes at least all of the following:
(i) The role of a pharmacy technician in the product verification process.
(ii) The legal requirements and liabilities of a final verification technician.
(iii) The use of technology assisted verification systems.
(iv) The primary causes of medication errors and misfills.
(v) The identification and resolution of dispensing errors.
(C) The practice setting where a licensed pharmacy technician performs final product
verification has in place policies and procedures including a quality assurance plan governing pharmacy technician technology-assisted final product verification.
(D) The technology enabled verification system must document and electronically record each step of the prescription process including which individuals complete each step.
(E) A licensed pharmacy technician shall not perform technology-assisted final product
verification for sterile or nonsterile compounding.
(F) Technology-assisted final product verification by a licensed pharmacy technician is not limited to a practice setting.
(G) A pharmacist using professional judgment may choose to delegate technology-assisted
final product verification after ensuring licensed pharmacy technicians have completed and documented relevant training or work experience.
(c) Access the electronic database of a pharmacy from inside or outside of the pharmacy to perform the delegated tasks in paragraph (iii) of this subdivision related to prescription
processing functions outside of the personal charge of a pharmacist.
(i) A pharmacy technician remotely performing the tasks in paragraph (iii) of this subdivision must be supervised by a licensed pharmacist.
(ii) The remote supervision in paragraph (i) of this subdivision means that a pharmacist directs and controls the actions of the remote technician using technology to ensure the supervising pharmacist does both of the following:
(A) Is readily and continuously available to answer questions, review the practice of the
supervised pharmacy technician, provide consultation, review records, and educate the pharmacy technician in the performance of functions.
(B) Has established predetermined procedures and drug protocol governing any activity
performed remotely including protection of patient confidentiality.
(iii) Delegated tasks relating to prescription processing functions include, but are not limited to, the following:
(A) Verification of a patient’s medication history.
(B) Data entry regarding processing prescription data and patient data.
(C) Claims adjudication.
(D) Handling phone calls regarding processing prescription data and patient data.
(E) Processing refill requests.
(F) Technology-assisted final product verification.
(G) Transferring prescriptions for non-controlled substances.
History: 2016 AACS; 2021 AACS; 2023 MR 19, Eff. Oct. 2, 2023.
Our Mission
The Emily Jerry Foundation is determined to help make our nation’s, world renowned, medical facilities safer for everyone, beginning with our babies and children. We are accomplishing this very important objective by focusing on increasing public awareness of key patient safety related issues and identifying technology and best practices that are proven to minimize the “human error” component of medicine. Through our ongoing efforts The Emily Jerry Foundation is working hard to save lives every day.
Recent Posts
Archives