
Happy Heavenly 22nd Birthday Emily + Upcoming Pacific Coast Patient Safety Conference
February 24, 2026
By ejfadmin
Tomorrow, I’m really looking forward to heading to Monterey, California to speak on behalf of the Emily Jerry Foundation at the Pacific Coast Patient Safety Conference, hosted by the California Society of Health – System Pharmacists. But today… Read More



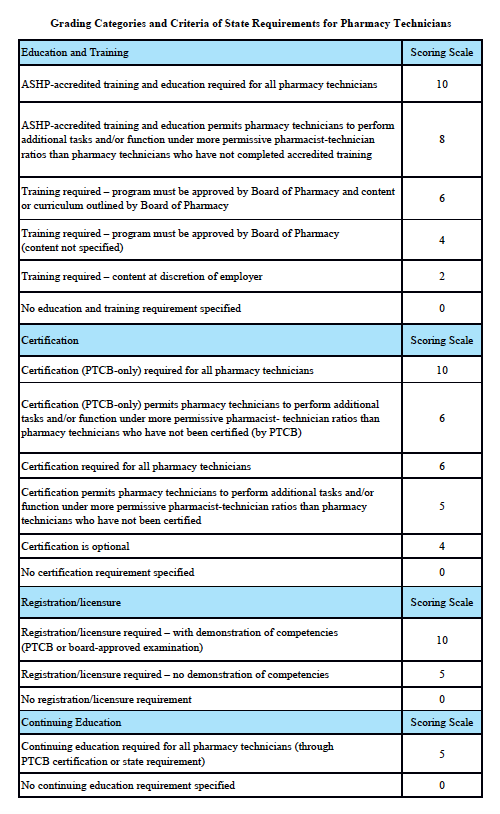
Nebraska Scorecard
Grading Scale:
A – 85-100%, B – 70-84.9%, C – 55-69.9%, D – 40-54.9%, F – 0-39.9%
Grading Categories & Criteria
Nebraska Revised Statutes
https://nebraskalegislature.gov/laws/statutes.php?statute=38-2836
38-2836.
Pharmacy technician, defined.
Pharmacy technician means an individual registered under sections 38-2890 to 38-2897.
Source
Laws 2007, LB247, § 80;
Laws 2007, LB463, § 932.
Pharmacy technicians; registration; requirements; certification.
(1) All pharmacy technicians employed by a health care facility licensed under the Health Care Facility Licensure Act shall be registered with the Pharmacy Technician Registry created in section 38-2893. In order to be employed as a pharmacy technician in such a health care facility, a pharmacy technician (a) shall be certified by a state or national certifying body which is approved by the board (i) by January 1, 2017, if the pharmacy technician was registered with the Pharmacy Technician Registry on January 1, 2016, or (ii) within one year after being registered with the Pharmacy Technician Registry, if the pharmacy technician was so registered after January 1, 2016, and (b) upon being so certified, shall maintain current certification during the time the pharmacy technician is so registered.
(2) To register as a pharmacy technician, an individual shall (a) be at least eighteen years of age, (b) be a high school graduate or be officially recognized by the State Department of Education as possessing the equivalent degree of education, (c) not have been convicted of any nonalcohol, drug-related felony, (d) not have been convicted of any nonalcohol, drug-related misdemeanor within five years prior to application, (e) file an application with the Division of Public Health of the Department of Health and Human Services, and (f) pay the applicable fee.
Source
Laws 2007, LB236, § 31;
R.S.Supp.,2007, § 71-1,147.65;
Laws 2015, LB37, § 51;
Laws 2016, LB680, § 1;
Laws 2024, LB1215, § 17.
38-2891.
Pharmacy technicians; authorized functions and tasks.
(1) A pharmacy technician shall only perform tasks which do not require the professional judgment of a pharmacist and which are subject to verification to assist a pharmacist in the practice of pharmacy.
(2) A pharmacy technician may administer vaccines, and such administration shall not be considered to be performing a task requiring the professional judgment of a pharmacist, when:
(a) The vaccines are verified by the pharmacist responsible for the supervision and verification of the activities of the pharmacy technician prior to administration;
(b) Administration is limited to intra-muscular in the deltoid muscle or subcutaneous on the arm to a person three years of age or older;
(c) The pharmacy technician is certified as required by section 38-2890;
(d) The pharmacy technician has completed certificate training in vaccine administration that includes, at a minimum, vaccine administration, blood-borne pathogen exposure, safety measures during administration, and biohazard handling;
(e) The pharmacy technician is currently certified in basic life-support skills for health care providers as determined by the board; and
(f) The pharmacist responsible for the supervision and verification of the activities of the pharmacy technician is on site.
(3) The functions and tasks which shall not be performed by pharmacy technicians include, but are not limited to:
(a) Receiving oral medical orders from a practitioner or his or her agent except as otherwise provided in subsection (4) of section 38-2870;
(b) Providing patient counseling;
(c) Performing any evaluation or necessary clarification of a medical order or performing any functions other than strictly clerical functions involving a medical order;
(d) Supervising or verifying the tasks and functions of pharmacy technicians;
(e) Interpreting or evaluating the data contained in a patient’s record maintained pursuant to section 38-2869;
(f) Releasing any confidential information maintained by the pharmacy;
(g) Performing any professional consultations; and
(h) Drug product selection, with regard to an individual medical order, in accordance with the Nebraska Drug Product Selection Act.
(4) The director shall, with the recommendation of the board, waive any of the limitations in subsection (2) of this section for purposes of a scientific study of the role of pharmacy technicians approved by the board. Such study shall be based upon providing improved patient care or enhanced pharmaceutical care. Any such waiver shall state the length of the study and shall require that all study data and results be made available to the board upon the completion of the study. Nothing in this subsection requires the board to approve any study proposed under this subsection.
Source
Laws 2007, LB236, § 32;
R.S.Supp.,2007, § 71-1,147.66;
Laws 2007, LB247, § 82;
Laws 2018, LB731, § 75;
Laws 2021, LB583, § 6;
Laws 2023, LB227, § 56.” “Consider adding:
38-2891.01.
Pharmacy technician; validate acts, tasks, and functions of pharmacy technician; policies and procedures.
(1) A pharmacy technician may validate the acts, tasks, and functions of another pharmacy technician only if:
(a) Both pharmacy technicians are certified by a state or national certifying body which is approved by the board;
(b) Both certified pharmacy technicians are working within the confines of a hospital preparing medications for administration in the hospital;
(c) Using bar code technology, radio frequency identification technology, or similar technology to validate the accuracy of medication;
(d) Validating medication that is prepackaged by the manufacturer or prepackaged and verified by a pharmacist; and
(e) Acting in accordance with policies and procedures applicable in the hospital established by the pharmacist in charge.
(2) The pharmacist in charge in a hospital shall establish policies and procedures for validation of medication by two or more certified pharmacy technicians before such validation process is implemented in the hospital.
Source
Laws 2019, LB74, § 5.
38-2892.
Pharmacy technicians; responsibility for supervision and performance.
(1) The pharmacist in charge of a pharmacy, remote dispensing pharmacy, or hospital pharmacy employing pharmacy technicians shall be responsible for the supervision and performance of the pharmacy technicians.
(2) Except as otherwise provided in the Automated Medication Systems Act, the supervision of pharmacy technicians at a pharmacy shall be performed by the pharmacist who is on duty in the facility with the pharmacy technicians or located in pharmacies that utilize a real-time, online database and have a pharmacist in all pharmacies. The supervision of pharmacy technicians at a remote dispensing pharmacy or hospital pharmacy shall be performed by the pharmacist assigned by the pharmacist in charge to be responsible for the supervision and verification of the activities of the pharmacy technicians.
Source
Laws 2007, LB236, § 33;
R.S.Supp.,2007, § 71-1,147.67;
Laws 2015, LB37, § 52;
Laws 2017, LB166, § 17;
Laws 2018, LB731, § 76.
38-2893.
Pharmacy Technician Registry; created; contents.
(1) The Pharmacy Technician Registry is created. The department shall list each pharmacy technician registration in the registry. A listing in the registry shall be valid for the term of the registration and upon renewal unless such listing is refused renewal or is removed as provided in section 38-2894.
(2) The registry shall contain the following information on each individual who meets the conditions set out in section 38-2890: (a) The individual’s full name; (b) information necessary to identify the individual; and (c) any other information as the department may require by rule and regulation.
Source
Laws 2007, LB236, § 34;
R.S.Supp.,2007, § 71-1,147.68;
Laws 2009, LB288, § 2.
38-2894.
Pharmacy technician; registration; disciplinary measures; procedure; Licensee Assistance Program; participation.
(1) A registration to practice as a pharmacy technician may be denied, refused renewal, removed, or suspended or have other disciplinary measures taken against it by the department, with the recommendation of the board, for failure to meet the requirements of or for violation of any of the provisions of subdivisions (1) through (18) and (20) through (27) of section 38-178 and sections 38-2890 to 38-2897 or the rules and regulations adopted under such sections.
(2) If the department proposes to deny, refuse renewal of, or remove or suspend a registration, it shall send the applicant or registrant a notice setting forth the action to be taken and the reasons for the determination. The denial, refusal to renew, removal, or suspension shall become final thirty days after mailing the notice unless the applicant or registrant gives written notice to the department of his or her desire for an informal conference or for a formal hearing.
(3) Notice may be served by any method specified in section 25-505.01, or the department may permit substitute or constructive service as provided in section 25-517.02 when service cannot be made with reasonable diligence by any of the methods specified in section 25-505.01.
(4) Pharmacy technicians may participate in the Licensee Assistance Program described in section 38-175.
Source
Laws 2007, LB236, § 35;
R.S.Supp.,2007, § 71-1,147.69;
Laws 2007, LB247, § 83;
Laws 2009, LB288, § 3;
Laws 2019, LB449, § 5;
Laws 2022, LB752, § 23;
Laws 2023, LB574, § 13.
38-2895.
Pharmacy technician; discipline against supervising pharmacist; enforcement orders.
(1) If a pharmacy technician performs functions requiring professional judgment and licensure as a pharmacist or performs functions without supervision and verification and such acts are known to the pharmacist supervising the pharmacy technician or the pharmacist in charge or are of such a nature that they should have been known to a reasonable person, such acts may be considered acts of unprofessional conduct on the part of the pharmacist supervising the pharmacy technician or the pharmacist in charge pursuant to section 38-178, and disciplinary measures may be taken against such pharmacist supervising the pharmacy technician or the pharmacist in charge pursuant to the Uniform Credentialing Act.
(2) Acts described in subsection (1) of this section may be grounds for the department, with the recommendation of the board, to apply to the district court in the judicial district in which the pharmacy is located for an order to cease and desist from the performance of any unauthorized acts. On or at any time after such application the court may, in its discretion, issue an order restraining such pharmacy or its agents or employees from the performance of unauthorized acts. After a hearing the court shall either grant or deny the application. Such order shall continue until the court, after a hearing, finds the basis for such order has been removed.
Source
Laws 2007, LB236, § 36;
R.S.Supp.,2007, § 71-1,147.70;
Laws 2007, LB247, § 84;
Laws 2015, LB37, § 53.
38-2896.
Pharmacy technician; reapplication for registration; lifting of disciplinary sanction.
A person whose registration has been denied, refused renewal, removed, or suspended from the Pharmacy Technician Registry may reapply for registration or for lifting of the disciplinary sanction at any time in accordance with the rules and regulations adopted and promulgated by the department.
Source
Laws 2007, LB236, § 37;
R.S.Supp.,2007, § 71-1,147.71.
Duty to report impaired practitioner; immunity.
(1) The requirement to file a report under subsection (1) of section 38-1,125 shall not apply to pharmacist interns or pharmacy technicians, except that a pharmacy technician shall, within thirty days after having first-hand knowledge of facts giving him or her reason to believe that any person in his or her profession, or any person in another profession under the regulatory provisions of the department, may be practicing while his or her ability to practice is impaired by alcohol, controlled substances, or narcotic drugs, report to the department in such manner and form as the department may require. A report made to the department under this section shall be confidential. The identity of any person making such report or providing information leading to the making of such report shall be confidential.
(2) A pharmacy technician making a report to the department under this section, except for self-reporting, shall be completely immune from criminal or civil liability of any nature, whether direct or derivative, for filing a report or for disclosure of documents, records, or other information to the department under this section. The immunity granted under this section shall not apply to any person causing damage or injury by his or her willful, wanton, or grossly negligent act of commission or omission.
(3) A report submitted by a professional liability insurance company on behalf of a credential holder within the thirty-day period prescribed in this section shall be sufficient to satisfy the credential holder’s reporting requirement under this section.
(4) Persons who are members of committees established under the Health Care Quality Improvement Act, the Patient Safety Improvement Act, or section 25-12,123 or witnesses before such committees shall not be required to report under this section. Any person who is a witness before such a committee shall not be excused from reporting matters of first-hand knowledge that would otherwise be reportable under this section only because he or she attended or testified before such committee.
(5) Documents from original sources shall not be construed as immune from discovery or use in actions under this section.
Source
Laws 2007, LB236, § 38;
R.S.Supp.,2007, § 71-1,147.72;
Laws 2017, LB166, § 18.
Source: Laws 2007, LB236, § 38; R.S.Supp., 2007, § 71-1,147.72. Operative date December 1, 2008.
Our Mission
The Emily Jerry Foundation is determined to help make our nation’s, world renowned, medical facilities safer for everyone, beginning with our babies and children. We are accomplishing this very important objective by focusing on increasing public awareness of key patient safety related issues and identifying technology and best practices that are proven to minimize the “human error” component of medicine. Through our ongoing efforts The Emily Jerry Foundation is working hard to save lives every day.
Recent Posts
Archives