
Keynote at Mount Sinai Health System’s Medication Safety Together Summit
November 24, 2025
By ejfadmin
I was truly honored to represent the Emily Jerry Foundation last week at Mount Sinai Health System, where I had the privilege of delivering the keynote address to kick off their Medication Safety Together – An Interdisciplinary Summit… Read More



Ohio Scorecard
Grading Scale:
A – 85-100%, B – 70-84.9%, C – 55-69.9%, D – 40-54.9%, F – 0-39.9%
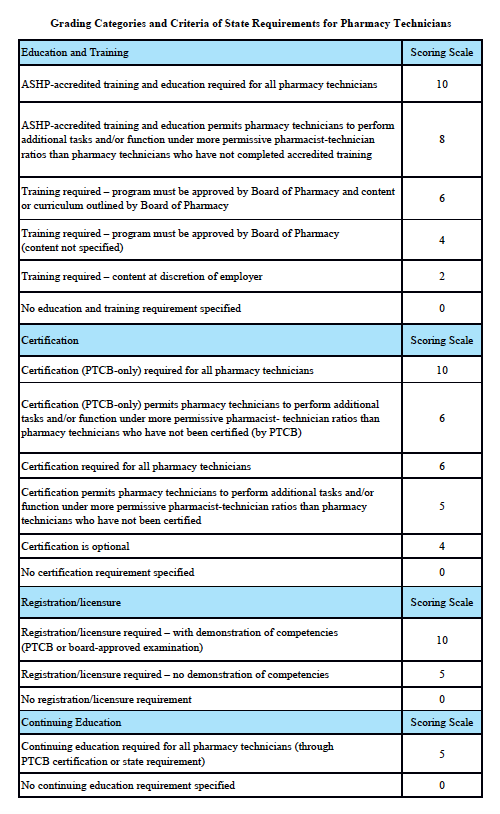
Grading Categories & Criteria
Appendix 1 – Statutes and Regulations
https://codes.ohio.gov/ohio-revised-code/chapter-4729
Section 4729.90 | Applicants for registration as registered pharmacy technician.
(A)(1) An applicant for registration as a registered pharmacy technician shall:
(a) Be at least eighteen years of age;
(b) Possess a high school diploma or a certificate of high school equivalence or have been employed continuously since prior to April 8, 2009, as a pharmacy technician without a high school diploma or certificate of high school equivalence;
(c) Comply with sections 4776.01 to 4776.04 of the Revised Code;
(d) Have successfully completed education and training that meets the requirements established by the board in rules adopted under section 4729.94 of the Revised Code.
(2) An applicant for registration as a certified pharmacy technician shall:
(a) Comply with divisions (A)(1)(a) and (c) of this section;
(b) Possess a high school diploma or a certificate of high school equivalence;
(c) Have successfully completed education and training that meets the requirements established by the board in rules adopted under section 4729.94 of the Revised Code;
(d) Have a current pharmacy technician certification from an organization that has been recognized by the board.
(B) A pharmacist or pharmacy intern whose license has been denied, revoked, suspended, or otherwise restricted by the board shall not be registered as a registered pharmacy technician or certified pharmacy technician.
Section 4729.901 | Form of application.
(A) An applicant for registration under section 4729.90 of the Revised Code shall file with the state board of pharmacy an application in the form and manner prescribed in rules adopted under section 4729.94 of the Revised Code. The application shall be accompanied by an application fee of fifty dollars, which shall not be returned if the applicant fails to qualify for registration.
(B) If the board is satisfied that the applicant meets the requirements of section 4729.90 of the Revised Code and any additional requirements established by the board and determines that the results of a criminal records check do not make the applicant ineligible, the board shall register the applicant as a registered pharmacy technician or certified pharmacy technician, as applicable.
(C) The board shall register as a registered pharmacy technician or certified pharmacy technician, as applicable, in accordance with Chapter 4796. of the Revised Code an applicant if either of the following applies:
(1) The applicant holds a license or is registered in another state.
(2) The applicant has satisfactory work experience, a government certification, or a private certification as described in that chapter as a pharmacy technician in a state that does not issue that license or registration.
(D) Registration under division (B) or (C) of this section is valid for the period specified by the board in rules adopted under section 4729.94 of the Revised Code. The period shall not exceed twenty-four months unless the board extends the period in the rules to adjust license renewal schedules.
Section 4729.902 | Registration renewal.
(A) A registered pharmacy technician or certified pharmacy technician shall file an application for registration renewal in the form and manner prescribed by the state board of pharmacy in rules adopted under section 4729.94 of the Revised Code. Registrations shall be renewed in accordance with the rules and the standard renewal procedure set forth in Chapter 4745. of the Revised Code. The renewal fee is twenty-five dollars per year.
(B)(1) A registered pharmacy technician or certified pharmacy technician who fails to renew registration in accordance with division (A) of this section is prohibited from engaging in the activities authorized by section 4729.91 of the Revised Code.
(2)(a) A registration that is not renewed by a date determined under division (A) of this section but has not lapsed for more than ninety days may be reinstated if the applicant does both of the following:
(i) Submits a renewal application in a form prescribed by the board in rules adopted under section 4729.94 of the Revised Code;
(ii) Pays the renewal fee and a late fee of fifty dollars.
(b) A registration that has lapsed for more than ninety days cannot be renewed, but the registration holder may reapply for registration.
Section 4729.91 | Permissible activities for registered pharmacy technician.
(A) A registered pharmacy technician may, under the direct supervision of a pharmacist, engage in the following activities at a location licensed as a terminal distributor of dangerous drugs to the extent that the activities do not require the exercise of professional judgment:
(1) Accepting new written or electronic prescription orders from a prescriber or a prescriber’s agent;
(2) Entering information into and retrieving information from a database or patient profile;
(3) Preparing and affixing labels;
(4) Stocking dangerous drugs and retrieving those drugs from inventory;
(5) Counting and pouring dangerous drugs into containers;
(6) Placing dangerous drugs into patient storage containers;
(7) Non-sterile drug compounding as authorized by the state board of pharmacy in rules adopted under section 4729.94 of the Revised Code;
(8) Other activities specified by the board in rules adopted under section 4729.94 of the Revised Code.
(B) A certified pharmacy technician may, under the direct supervision of a pharmacist, engage in the following activities at a location licensed as a terminal distributor of dangerous drugs to the extent that the activities do not require the exercise of professional judgment:
(1) Any activity listed in division (A) of this section;
(2) Accepting or requesting refill authorizations for dangerous drugs that are not controlled substances from a prescriber or the prescriber’s agent, so long as there is no change from the original prescription;
(3) S terile and non-sterile drug compounding as authorized by the board in rules adopted under section 4729.94 of the Revised Code;
(4) Other activities specified by the board in rules adopted under section 4729.94 of the Revised Code.
Section 4729.94 | Rules.
The state board of pharmacy shall adopt rules under section 4729.26 of the Revised Code governing registration of registered pharmacy technicians, certified pharmacy technicians, and pharmacy technician trainees. The rules shall include all of the following:
(A) Application and renewal forms and procedures;
(B) Reapplication forms and procedures for individuals whose registration has lapsed more than ninety days;
(C) Education and training requirements, requirements for employer-administered training programs, and other requirements considered appropriate by the board;
(D) Additional activities permitted by divisions (A)(7) and (B)(4) of section 4729.91 of the Revised Code;
(E) Requirements for sterile and non-sterile drug compounding ;
(F) Continuing education requirements;
(G) Conduct that constitutes dishonesty or unprofessional conduct by a registered pharmacy technician, certified pharmacy technician, or pharmacy technician trainee;
(H) Additional conduct for which the board may impose discipline under section 4729.96 of the Revised Code on a registered pharmacy technician, certified pharmacy technician, or pharmacy technician trainee;
(I) Any other rules the board considers appropriate to implement sections 4729.90 to 4729.96 of the Revised Code.
Section 4729.95 | Violations.
(A) No person who is not a pharmacist, pharmacy intern, registered pharmacy technician, certified pharmacy technician, or pharmacy technician trainee shall knowingly engage in any of the activities listed in section 4729.91 of the Revised Code in a location licensed as a terminal distributor of dangerous drugs or while performing the function of a terminal distributor, except that this division does not prevent a licensed health care professional from engaging in activities that are authorized by law as part of the licensed professional’s practice.
(B) No pharmacist shall knowingly allow any person employed or otherwise under the control of the pharmacist to violate division (A) of this section.
(C) No terminal distributor of dangerous drugs shall knowingly allow any person employed or otherwise under the control of the person who owns, manages, or conducts the terminal distributor to violate division (A) of this section.
Ohio Administrative Code
https://www.pharmacy.ohio.gov/LawsRules/OAC
Rule 4729:3-1-01 | Definitions – pharmacy technicians.
(E) “”Certified pharmacy technician”” means a person who:
(1) Has completed an approved training program pursuant to rule 4729:3-3-02 of the Administrative Code or has complied with the reciprocity requirements of 4729:3-2-01 of the Administrative Code;
(2) Is registered with the state board of pharmacy;
(3) Practices in this state in accordance with rule 4729:3-3-04 of the Administrative Code; and
(4) Maintains a current pharmacy technician certification from an organization that has been recognized by the board.
(O) “”Pharmacy technician trainee”” means a person who:
(1) Intends to enroll or is enrolled in an approved training program pursuant to rule 4729:3-3-02 of the Administrative Code to obtain a registration as a registered or certified pharmacy technician;
(2) Is registered as a pharmacy technician trainee with the state board of pharmacy; and
(3) Practices in this state in accordance with rule 4729:3-3-01 of the Administrative Code.” “Rule 4729:3-2-01 | Registration procedures.
(A) An applicant for registration as a pharmacy technician trainee shall:
(1) Comply with all requirements set forth in section 4729.92 of the Revised Code.
(2) Comply with the criminal records check requirements pursuant to rule 4729:3-2-02 of the Administrative Code.
(3) Submit a complete application for registration, in a manner determined by the board, that includes:
(a) The required application fee of twenty-five dollars, including any transaction fee as required by section 125.18 of the Revised Code.
(b) Documentation, as specified by the board, that the applicant meets the following requirements:
(i) Has a high school diploma, a certificate of high school equivalence, a foreign school diploma that is equivalent to a U.S. high school diploma or has been employed continuously since prior to April 8, 2009, as a pharmacy technician without a high school diploma or certificate of high school equivalence;
(ii) Is at least eighteen years of age.
(c) Notwithstanding the requirements of paragraph (A)(3)(b) of this rule, the board may register as a pharmacy technician trainee an applicant who is seventeen or eighteen years of age and does not possess a high school diploma or certificate of high school equivalence if the applicant is enrolled in a career-technical school program that is approved by the board and conducted by a city, exempted village, local, or joint vocational school district.
(d) Any additional information or documentation as determined by the board.
(4) A pharmacy technician trainee licensed or registered in another state may apply for registration by reciprocity by complying with the requirements listed in paragraphs (A)(1) to (A)(3) of this rule.
(B) An applicant for registration as a registered pharmacy technician shall:
(1) Comply with all requirements set forth in section 4729.90 of the Revised Code.
(2) Comply with either of the following:
(a) Have completed an approved training program pursuant to rule 4729:3-3-02 of the Administrative Code; or
(b) Hold a pharmacy technician registration or license issued by another state and have actively worked as a pharmacy technician for at least one year within the previous five years of application.
(3) Comply with the criminal records check requirements pursuant to rule 4729:3-2-02 of the Administrative Code.
(4) Submit a complete application for registration, in a manner determined by the board, that includes:
(a) The required application fee of fifty dollars, including any transaction fee as required by section 125.18 of the Revised Code;
(b) Except for applicants currently registered as pharmacy technician trainees, documentation, as specified by the board, that the applicant meets the following requirements:
(i) Has a high school diploma, a certificate of high school equivalence, a foreign school diploma that is equivalent to a U.S. high school diploma or has been employed continuously since prior to April 8, 2009, as a pharmacy technician without a high school diploma or certificate of high school equivalence;
(ii) Is at least eighteen years of age; and
(iii) If the applicant has a foreign school diploma that is equivalent to a U.S. high school diploma, the applicant shall submit evidence of successful completion of the “”Test of English as a Foreign Language, Internet-based test”” (TOEFL iBT) pursuant to rule 4729:3-2-05 of the Administrative Code.
(c) Paragraph (B)(4)(b)(iii) of this rule shall not apply if the applicant complies with any of the following:
(i) Submits a diploma or transcript demonstrating completion of an associate degree or higher from an accredited college, junior college, community college, or university in the United States.
(ii) Submits verification of active professional license or registration issued under the following chapters of the Revised Code: 4715., 4723., 4725., 4729., 4730., 4731., 4732., 4734., 4741., 4744., 4753., 4755., 4757., 4759., 4760., 4761., 4762., 4774., 4778., 4779., 4783.
(iii) Submits verification of an active professional license or registration from another state that permits the applicant to engage in the same profession, occupation, or occupational activity as any license or registration issued by an agency listed in paragraph (B)(4)(c)(ii) of this rule.
(iv) Submits an attestation signed by the responsible person, or the equivalent in the state where the technician is registered, of the pharmacy where the technician is actively employed or was employed in the five years prior to the date of submission of an application. The responsible person must complete the required attestation form provided by the board and attest to their personal observation that the technician applicant demonstrates the required proficiency (reading, listening, speaking, and writing) in the english language to practice safely and effectively as a registered pharmacy technician.
(v) Submits documentation of any other board approved method for demonstrating english language proficiency.
(d) Any of the following documentation:
(i) An attestation, certificate of completion, or other board approved documentation that the applicant has successfully completed an approved training program in accordance with rule 4729:3-3-02 of the Administrative Code.
(ii) Documentation, as determined by the board, demonstrating compliance with the reciprocity requirements of paragraph (B)(2)(b) of this rule.
(e) Any additional information or documentation as determined by the board.
(C) An applicant for registration as a certified pharmacy technician shall:
(1) Comply with all requirements set forth in section 4729.90 of the Revised Code.
(2) Comply with either of the following:
(a) Have completed an approved training program pursuant to rule 4729:3-3-02 of the Administrative Code;
(b) Hold a pharmacy technician registration or license issued by another state and have actively worked as a pharmacy technician for at least one year within the previous five years of application; or
(c) Holds a current pharmacy technician certification from an organization that has been recognized by the board for at least two years immediately preceding the date the application is submitted and has been actively practicing as a pharmacy technician in a state that does not issue a pharmacy technician license or registration for at least two of the five years immediately preceding the date the application is submitted.
(3) Comply with the criminal records check requirements pursuant to rule 4729:3-2-02 of the Administrative Code.
(4) Submit a complete application for registration, in a manner determined by the board, that includes:
(a) The required application fee of fifty dollars, including any transaction fee as required by section 125.18 of the Revised Code, except as provided in rule 4729:3-2-03 of the Administrative Code;
(b) Documentation, as specified by the board, that the applicant has a current pharmacy technician certification from an organization that has been recognized by the board.
(c) Except for applicants currently registered as pharmacy technician trainees, documentation, as specified by the board, that the applicant meets the following requirements:
(i) Has a high school diploma, a certificate of high school equivalence or a foreign school diploma that is equivalent to a U.S. high school diploma;
(ii) Is at least eighteen years of age; and
(iii) If the applicant has a foreign school diploma that is equivalent to a U.S. high school diploma, the applicant shall submit evidence of successful completion of the “”Test of English as a Foreign Language, Internet-based test”” (TOEFL iBT) pursuant to rule 4729:3-2-05 of the Administrative Code.
(d) Paragraph (C)(4)(c)(iii) of this rule shall not apply if the applicant complies with either of the following:
(i) Submits a diploma or transcript demonstrating completion of an associate degree or higher from an accredited college, junior college, community college or university in the United States.
(ii) Submits verification of active professional license or registration issued under the following chapters of the Revised Code: 4715., 4723., 4725., 4729., 4730., 4731., 4732., 4734., 4741., 4744., 4753., 4755., 4757., 4759., 4760., 4761., 4762., 4774., 4778., 4779., 4783.
(iii) Submits verification of an active professional license or registration from another state that permits the applicant to engage in the same profession, occupation, or occupational activity as any license or registration issued by an agency listed in paragraph (C)(4)(d)(ii) of this rule.
(iv) Submits an attestation signed by the responsible person, or the equivalent in the state where the technician is registered, of the pharmacy where the technician is actively employed or was employed in the five years prior to the date of submission of an application. The responsible person must complete the required attestation form provided by the board and attest to their personal observation that the technician applicant demonstrates the required proficiency (reading, listening, speaking, and writing) in the english language to practice safely and effectively as a certified pharmacy technician.
(v) Submits documentation of any other board approved method for demonstrating english language proficiency.
(e) Any of the following documentation:
(i) An attestation, certificate of completion, or other board approved documentation that the applicant has successfully completed an approved training program in accordance rule 4729:3-3-02 of the Administrative Code.
(ii) Documentation, as determined by the board, demonstrating compliance with the reciprocity requirements of paragraphs (C)(2)(b) and (C)(2)(c) of this rule.
(f) Any additional information or documentation as determined by the board.
(D) Pursuant to section 4729.921 of the Revised Code, a registration for a pharmacy technician trainee is valid for eighteen months from the date of issuance.
(E) A pharmacy technician trainee that fails to meet the education and training requirements during the trainee’s initial registration period, may apply for reinstatement of their registration by submitting an application and required fee as required by paragraph (A) of this rule.
(F) Pursuant to section 4729.96 of the Revised Code, a limited or restricted registration may be issued to an applicant upon the determination of the board.
(G) An initial registration for a registered pharmacy technician and certified pharmacy technician is valid until the renewal date set forth in rule 4729:3-2-03 of the Administrative Code.
(H) Failure to complete all application requirements within thirty days after being notified by the board may result in the application being deemed abandoned as defined in rule 4729:3-1-01 of the Administrative Code.
(I) Registration fees for veterans shall be waived upon submission of the appropriate documentation. Documentation required to obtain a fee waiver will be published on the state board of pharmacy’s web site: www.pharmacy.ohio.gov.
Rule 4729:3-2-03 | Renewal procedures.
(A) A registered pharmacy technician shall submit a renewal application, in a manner determined by the board, that includes all the following:
(1) An attestation that the technician has completed the specified continuing education requirements pursuant to rule 4729:3-5-01 of the Administrative Code.
(2) The required biennial fee of fifty dollars, except as provided in paragraph (A)(3) of this rule.
(3) A registered pharmacy technician may apply to be a certified pharmacy technician at no cost if the registered technician applies for a certified pharmacy technician registration during the renewal application period established in paragraph (C) of this rule.
(4) Any additional information or documentation as determined by the board.
(B) A certified pharmacy technician shall submit a renewal application, in a manner determined by the board, that includes all the following:
(1) An attestation that the technician has valid technician certification number from an organization that has been recognized by the board and has complied with the continuing education requirements of that organization.
(2) The required biennial fee of fifty dollars.
(3) Any additional information or documentation as determined by the board.
(C) A renewal application for registered pharmacy technicians shall be submitted no later than the thirty first of March of each even-numbered year. Renewal applications shall be accepted no earlier than January of each even-numbered year on a date determined by the board’s director of licensing.
An initial registration issued by the state board of pharmacy on or after the first of December of every odd-numbered year in accordance with Chapter 4729. of the Revised Code entitles the individual to whom it is issued to practice as a registered pharmacy technician until the renewal date immediately following the next required renewal date.
(D) A renewal application for certified pharmacy technicians shall be submitted no later than the thirtieth of September of each even-numbered year. Renewal applications shall be accepted no earlier than July of each even-numbered year on a date determined by the board’s director of licensing.
An initial registration issued by the state board of pharmacy on or after the first of June of every even-numbered year in accordance with Chapter 4729. of the Revised Code entitles the individual to whom it is issued to practice as a certified pharmacy technician until the renewal date immediately following the next required renewal date.
(E) A registered pharmacy technician or certified pharmacy technician who fails to renew a registration in accordance with this rule is prohibited from engaging in the activities authorized by section 4729.91 of the Revised Code and rules 4729:3-3-03 and 4729:3-3-04 of the Administrative Code.
(F) A registered pharmacy technician or certified pharmacy technician who fails to renew a registration in accordance with this rule, but has not lapsed for more than ninety days, may be renewed if the applicant complies with the following:
(1) Submits a renewal application in accordance with this rule;
(2) Pays the renewal fee and a late fee of fifty dollars.
(G) A registrant that fails to renew for more than ninety days may not renew a registration and the registration shall be considered expired and cannot be renewed. An individual may apply to reinstate a registration in accordance with rule 4729:3-2-01 of this rule.
(H)
(1) In accordance with section 5903.10 of the Revised Code, a holder of an expired registration shall be granted a renewal of the registration by the state board of pharmacy at the usual cost without penalty and without need to meet the requirements set forth in rule 4729:3-2-01 of the Administrative Code if not otherwise disqualified because of mental or physical disability and if either of the following applies:
(a) The registration was not renewed because of the holder’s service in the armed forces of the United States or a reserve component of the armed forces.
(b) The registration was not renewed because the holder’s spouse served in the armed forces of the United States or a reserve component of the armed forces.
(2) A registered or certified pharmacy technician shall submit proper documentation certifying the active duty service and the length of that active duty service. Documentation required to obtain a renewal pursuant to paragraph (H)(1) of this rule will be published on the state board of pharmacy’s web site: www.pharmacy.ohio.gov.
(I) Renewal fees for veterans shall be waived upon submission of the appropriate documentation. Documentation required to obtain a fee waiver will be published on the state board of pharmacy’s web site: www.pharmacy.ohio.gov.
Rule 4729:3-2-08 | Verification of registration and certification.
(A) In accordance with section 4729.95 of the Revised Code, a terminal distributor of dangerous drugs shall develop and implement a policy to ensure persons holding a technician trainee, registered technician, or certified pharmacy technician registration hold an active registration in accordance with this division of the Administrative Code. The policy shall require, at a minimum, the following:
(1) Documented verification of a technician trainee, registered technician, or certified pharmacy technician registration prior to commencing employment with the terminal distributor of dangerous drugs;
(2) Documented verification of registration, to be conducted at least every twenty-four months, of every technician trainee, registered technician, and certified pharmacy technician employed by the terminal distributor of dangerous drugs; and
(3) Documented verification, to be conducted at least every twenty-four months, of pharmacy technician certification from an organization that has been recognized by the board for all certified pharmacy technicians employed by the terminal distributor of dangerous drugs.
(B) Documented verifications shall be maintained for a period of three years from the date of verification.
Rule 4729:3-3-02 | Approved pharmacy technician training programs.
The purpose of this rule is to set standards for pharmacy technician training programs to ensure that graduates of the programs have the basic knowledge and experience in general pharmacy to practice in most pharmacy settings.
(A) The state board of pharmacy hereby approves the following pharmacy technician training programs:
(1) A pharmacy technician training program that holds a current accreditation from the American society of health-system pharmacists/accreditation council for pharmacy education.
(2) A program of training for pharmaceutical technicians conducted by a branch of the Armed Forces of the United States, the Indian health service of the United States department of health and human services, or the United States department of veterans affairs.
(3) An employer-based training program that meets the requirements in paragraph (B) of this rule.
(4) A pharmacy technician training program offered by an Ohio public high school as part of a career-technical education program approved by the Ohio department of education pursuant to section 3317.161 of the Revised Code. Each career-technical education program shall require approval by the state board of pharmacy in accordance with standards adopted by the board. Instructions for obtaining board approval will be made available on the board’s website: www.pharmacy.ohio.gov.
(5) Successful completion of a doctor of pharmacy (PharmD) program from an approved school of pharmacy in accordance with rule 4729-5-01 of the Administrative Code if the applicant’s graduation date is within five years of an application for registration.
(6) Held an active pharmacist or pharmacy intern license or registration in good standing from a licensing agency of any state or jurisdiction for at least one year within five years of an application for registration.
(B) An employer-based training program shall comply with all the following:
(1) The program shall have a program director. The program director and the employer licensed as a terminal distributor of dangerous drugs shall be accountable for the overall quality of the employer-based training program.
The program director shall be a licensed pharmacist in this or any other state that is in good standing.
(2) The program shall consist of didactic and practical experience training, as follows:
(a) A didactic training component that includes, at a minimum, instruction in all of the following:
(i) Mathematical calculations essential to the duties of a pharmacy technician;
(ii) Federal and state laws, rules, and regulations that affect pharmacy practice, including specific laws, rules, and regulations which address the use of technicians;
(iii) Medical and pharmaceutical terminology, symbols, and abbreviations used in the practice of pharmacy and components of a prescription;
(iv) Preparation, packaging, labeling, and proper storage of dangerous drugs;
(v) Knowledge and skills in areas of science relevant to the pharmacy technician’s role, including pharmacology;
(vi) Medication safety and error prevention;
(vii) Maintaining confidentiality of patient information, including the Patient Rights and Health Insurance Portability and Accountability Act (HIPAA);
(viii) Ethical and professional standards of practice;
(ix) Recordkeeping and inventory control; and
(x) Patient and caregiver communication, including communicating with diverse populations.
(b) Didactic training may include self-directed learning experiences, including but not limited to home study, computer programs, internet or web-based courses, or any other coursework approved by the program director.
(c) The program shall ensure the required didactic training evaluates a participant’s knowledge of the topics listed in paragraph (B)(2)(a) of this rule. The evaluation must include an examination consisting of a minimum of ninety questions in accordance with testing guidelines adopted by the board. The employer shall have procedures that ensure the security and integrity of the examination materials, describe the testing format, and define the successful completion of an examination, which must be at least seventy-five per cent. The examination shall consist primarily of multiple choice, essay, or short answer questions. The questions on the examination shall not be given to the examinee prior to taking the examination. The answers to the examination must not be given to the examinee prior to or during the examination. The examinee shall agree in writing not to share the questions or answers to the examination with any other person.
(d) The didactic training requirements in paragraph (B)(2)(a) of this rule are waived if the trainee has a current pharmacy technician certification from an organization that has been recognized by the board.
(e) Three hundred hours of practical experience in a pharmacy under the direct supervision of a licensed pharmacist that directly relates to the activities permitted in paragraph (B) of rule 4729:3-3-01 of the Administrative Code.
(3) A written or electronic record of training and education shall be maintained as part of the training program that documents the completion of the training requirements, including the number of practical experience hours completed.
(4) It shall be the responsibility of the terminal distributor of dangerous drugs providing employer-based training to ensure that the documentation provided to pharmacy technician trainees accurately reflects the completion of an approved training program. Failure on the part of the terminal distributor of dangerous drugs to ensure the accuracy of training documentation shall be considered a violation of rule 4729:5-4-01 of the Administrative Code.
(5) The program director must ensure that regular and ongoing assessments of program effectiveness are conducted and use the evaluations for continuous improvement of the program. Measures shall include, but are not limited to, the following:
(a) Program completion; and
(b) Program participant satisfaction.
(6) The program shall maintain the following records for a minimum of three years and shall be furnished to the state board of pharmacy within three business days of receipt of a request from the board:
(a) All technician training records and evaluations;
(b) Program assessments conducted in accordance with this rule.
(7) Employer-based training programs may be subject to audit to ensure compliance with the requirements of this rule.
(a) An employer-based training program subject to audit shall provide all requested documentation demonstrating compliance with this rule within thirty days of a request by the board.
(b) Unless an extension is granted, failure to provide the requested documentation within thirty days of a request by the board may result in the suspension of the approval status of the training program.
(c) After reviewing the training program, the board may return it to the employer for revision. Failure to make the necessary revisions may result in the suspension of the approval status of the training program.
(d) The approval status of a training program may be reinstated only after the employer meets the requirements of this rule and any additional requirements as determined by the board.
(C) In order to perform non-sterile drug compounding, a pharmacy technician trainee shall complete the following training requirements prior to compounding non-sterile preparations:
(1) Training shall comply with the requirements set forth in the United States pharmacopeia chapter <795>, as defined in rule 4729:7-1-01 of the Administrative Code.
(2) If preparing non-sterile hazardous compounded drugs in accordance with rule 4729:7-2-03 of the Administrative Code, the training shall also comply with the applicable requirements set forth in United States pharmacopeia chapter <800>, as defined in rule 4729:7-1-01 of the Administrative Code.
(3) If preparing non-sterile radiopharmaceuticals in accordance with rule 4729:5-6-03 of the Administrative Code, the training shall also comply with the applicable requirements set forth in United States pharmacopeia chapter <825>, as defined in rule 4729:5-6-01 of the Administrative Code.
(4) Non-sterile drug compounding training shall be obtained through completion of a site-specific, structured on-the-job didactic and experiential training program and shall not be transferable to another practice site, except between practice sites under common ownership and control.
(5) When the responsible person or a pharmacist designated by the responsible person is satisfied with the employee’s knowledge and proficiency, the responsible person or the responsible person’s designee will sign the documentation records to show that the employee was appropriately trained in accordance with this paragraph.
(6) Ensuring pharmacy technician trainees are properly trained shall be the responsibility of the terminal distributor of dangerous drugs and the licensee’s responsible person.
(7) All training requirements set forth in this paragraph shall be appropriately documented and made readily retrievable for immediate inspection by an agent of the state board of pharmacy. Documentation shall be maintained by the terminal distributor of dangerous drugs for a minimum of three years.
(8) The training required pursuant to this paragraph may be used to meet the practical experience hours required in paragraph (B)(2)(e) of this rule.
(D) In order to perform sterile drug compounding, a pharmacy technician trainee shall complete the following training requirements prior to compounding sterile preparations:
(1) Training shall comply with the requirements set forth in the United States pharmacopeia chapter <797>, as defined in rule 4729:7-1-01 of the Administrative Code.
(2) If preparing sterile hazardous compounded drugs in accordance with rule 4729:7-2-03 of the Administrative Code, the training shall also comply with the applicable requirements set forth in United States pharmacopeia chapter <800>, as defined in rule 4729:7-1-01 of the Administrative Code.
(3) If preparing sterile radiopharmaceuticals in accordance with rule 4729:5-6-03 of the Administrative Code, the training shall also comply with the applicable requirements set forth in United States pharmacopeia chapter <825>, as defined in rule 4729:5-6-01 of the Administrative Code.
(4) Sterile drug compounding training shall be obtained through completion of a site-specific, structured on-the-job didactic and experiential training program and shall not be transferable to another practice site, except between practice sites under common ownership and control.
(5) When the responsible person or a pharmacist designated by the responsible person is satisfied with the employee’s knowledge and proficiency, the responsible person or the responsible person’s designee will sign the documentation records to show that the employee was appropriately trained in accordance with this paragraph.
(6) Ensuring pharmacy technician trainees are properly trained shall be the responsibility of the terminal distributor of dangerous drugs and the licensee’s responsible person.
(7) All training requirements set forth in this paragraph shall be appropriately documented and made readily retrievable for immediate inspection by an agent of the state board of pharmacy. Documentation shall be maintained by the terminal distributor of dangerous drugs for a minimum of three years.
(8) The training required pursuant to this paragraph may be used to meet the practical experience hours required in paragraph (B)(2)(e) of this rule.
(E) A terminal distributor of dangerous drugs and the licensee’s responsible person shall be responsible for the implementation of additional training that is of appropriate breadth and depth to clearly address the competencies for a technician trainee to safely and effectively work in a specific practice setting.
(F) Unless otherwise approved by the board, a board approved training program is only valid for application as a registered pharmacy technician or certified pharmacy technician in accordance with rule 4729:3-2-01 of the Administrative Code if the program was completed within five years of an application for registration.
(G) Paragraph (F) of this rule does not apply in the following circumstances:
(1) An applicant for registration has been actively practicing as a pharmacy technician in this or another state within one year of application to the board; or
(2) An applicant has maintained a current pharmacy technician certification from an organization that has been recognized by the board.
(H) An individual may sit for an examination to obtain a pharmacy technician certification from an organization that has been recognized by the board at any time.
Rule 4729:3-3-03 | Registered pharmacy technicians.
(A) A registered pharmacy technician shall wear a name tag or badge which contains the designation “”Registered Pharmacy Technician.”” The required designation may be added to an existing name tag or badge. The name tag or badge and the required designation shall contain lettering of a legible size.
(B) A registered pharmacy technician may, under the direct supervision of a pharmacist, engage in the following activities at a location licensed as a terminal distributor of dangerous drugs to the extent that the activities do not require the exercise of professional judgment:
(1) Accepting new written, faxed or electronic prescription orders from a prescriber or a prescriber’s agent but shall not include verbal orders;
(2) Requesting refill authorizations for dangerous drugs from a prescriber or prescriber’s agent, so long as there is no change from the original prescription;
(3) Entering information into and retrieving information from a database or patient profile;
(4) Preparing and affixing labels;
(5) Stocking dangerous drugs and retrieving those drugs from inventory;
(6) Counting and pouring dangerous drugs into containers;
(7) Placing dangerous drugs into containers prior to dispensing by a pharmacist;
(8) Non-sterile drug compounding in accordance with the required training in paragraph (D) of this rule;
(9) Sterile drug compounding in accordance with the required training in paragraph (E) of this rule;
(10) Packaging and selling a dangerous drug to a patient or patient representative;
(11) Sending or receiving electronic prescriptions between pharmacies accessing the same prescription records in a centralized database or pharmacy computers linked in any other manner;
(12) Stocking automated drug storage systems, floor stock, and crash carts at a location licensed as a terminal distributor of dangerous drugs if either of the following applies:
(a) The terminal distributor utilizes barcode administration for restocking the drugs and develops and implements a quality assurance program to ensure the accuracy of the personnel stocking the dangerous drugs; or
(b) For restocking automated drug storage systems only: a pharmacist verifies the final dispensing of a dangerous drug removed from the automated drug storage system.
(C) A registered pharmacy technician may:
(1) Engage in remote entry of prescriptions in accordance rule 4729:5-5-25 of the Administrative Code; or
(2) Engage in remote entry of medication orders in accordance with rule 4729:5-9-02.15 of the Administrative Code.
(D) In order to perform non-sterile drug compounding, a registered pharmacy technician shall complete the following training requirements prior to compounding non-sterile preparations:
(1) Training shall comply with the requirements set forth in the United States pharmacopeia chapter <795>, as defined in rule 4729:7-1-01 of the Administrative Code.
(2) If preparing non-sterile hazardous compounded drugs in accordance with rule 4729:7-2-03 of the Administrative Code, the training shall also comply with the applicable requirements set forth in United States pharmacopeia chapter <800>, as defined in rule 4729:7-1-01 of the Administrative Code.
(3) If preparing non-sterile radiopharmaceuticals in accordance with rule 4729:5-6-03 of the Administrative Code, the training shall also comply with the applicable requirements set forth in United States pharmacopeia chapter <825>, as defined in rule 4729:5-6-01 of the Administrative Code.
(4) Non-sterile drug compounding training shall be obtained through completion of a site-specific, structured on-the-job didactic and experiential training program and shall not be transferable to another practice site, except between practice sites under common ownership and control.
(5) When the responsible person or a pharmacist designated by the responsible person is satisfied with the employee’s knowledge and proficiency, the responsible person or the responsible person’s designee will sign the documentation records to show that the employee was appropriately trained in accordance with this rule.
(6) Ensuring registered pharmacy technicians are properly trained shall be the responsibility of the terminal distributor of dangerous drugs and the licensee’s responsible person.
(7) All training requirements set forth in this paragraph shall be appropriately documented and made readily retrievable for immediate inspection by an agent of the state board of pharmacy. Documentation shall be maintained by the terminal distributor of dangerous drugs for a minimum of three years.
(E) In order to perform sterile drug compounding, a registered pharmacy technician shall complete the following training requirements prior to compounding sterile preparations:
(1) Training shall comply with the requirements set forth in the United States pharmacopeia chapter <797>, as defined in rule 4729:7-1-01 of the Administrative Code.
(2) If preparing sterile hazardous compounded drugs in accordance with rule 4729:7-2-03 of the Administrative Code, the training shall also comply with the applicable requirements set forth in United States pharmacopeia chapter <800>, as defined in rule 4729:7-1-01 of the Administrative Code.
(3) If preparing sterile radiopharmaceuticals in accordance with rule 4729:5-6-03 of the Administrative Code, the training shall also comply with the applicable requirements set forth in United States pharmacopeia chapter <825>, as defined in rule 4729:5-6-01 of the Administrative Code.
(4) Sterile drug compounding training shall be obtained through completion of a site-specific, structured on-the-job didactic and experiential training program and shall not be transferable to another practice site, except between practice sites under common ownership and control.
(5) When the responsible person or a pharmacist designated by the responsible person is satisfied with the employee’s knowledge and proficiency, the responsible person or the responsible persons designee will sign the documentation records to show that the employee was appropriately trained in accordance with this rule.
(6) Ensuring registered pharmacy technicians are properly trained shall be the responsibility of the terminal distributor of dangerous drugs and the licensee’s responsible person.
(7) All training requirements set forth in this paragraph shall be appropriately documented and made readily retrievable for immediate inspection by an agent of the state board of pharmacy. Documentation shall be maintained by the terminal distributor of dangerous drugs for a minimum of three years.
(F) A registered pharmacy technician shall be permitted to perform sterile compounding in accordance with this rule if all the following apply:
(1) The registered pharmacy technician is in the process of studying to obtain technician certification from an organization that has been recognized by the board;
(2) The registered pharmacy technician shall not engage in sterile drug compounding at the location licensed as a terminal distributor of dangerous drugs for longer than eighteen months from the date the technician completed the required sterile compounding training in paragraph (E) of this rule.
(3) A terminal distributor of dangerous drugs that permits a registered pharmacy technician to engage in sterile drug compounding shall develop and implement a process to ensure the registered pharmacy technician does not exceed the time limitation established in paragraph (F)(2) of this rule. The terminal distributor of dangerous drugs shall be accountable for a registered technician found to be in violation of this paragraph.
(G) A terminal distributor of dangerous drugs and the licensee’s responsible person shall be responsible for the implementation of policies and procedures for additional training appropriate to duties and responsibilities performed by a registered pharmacy technician as well as an ongoing quality assurance plan to ensure competency.” “Rule 4729:3-3-04 | Certified pharmacy technicians.
(A) As used in this rule, “”positive identification”” has the same meaning as in rule 4729:5-5-01 of the Administrative Code.
(B) A certified pharmacy technician shall wear a name tag or badge which contains the designation “”Certified Pharmacy Technician.”” The required designation may be added to an existing name tag or badge. The name tag or badge and the required designation shall contain lettering of a legible size.
(C) A certified pharmacy technician may, under the direct supervision of a pharmacist, engage in the following activities at a location licensed as a terminal distributor of dangerous drugs to the extent that the activities do not require the exercise of professional judgment:
(1) Accepting new written, faxed or electronic prescription orders from a prescriber or a prescriber’s agent. New verbal prescription orders from a prescriber or a prescriber’s agent for non-controlled drugs may be accepted pursuant to paragraph (C)(13) of this rule.
(2) Entering information into and retrieving information from a database or patient profile.
(3) Preparing and affixing labels.
(4) Stocking dangerous drugs and retrieving those drugs from inventory.
(5) Counting and pouring dangerous drugs into containers.
(6) Placing dangerous drugs into containers prior to dispensing by a pharmacist.
(7) Non-sterile drug compounding in accordance with the required training in paragraph (D) of this rule.
(8) Sterile drug compounding in accordance with the required training in paragraph (E) of this rule.
(9) Packaging and selling a dangerous drug to a patient or patient representative.
(10) Sending or receiving electronic prescriptions between pharmacies accessing the same prescription records in a centralized database or pharmacy computers linked in any other manner.
(11) Stocking automated drug storage systems, floor stock, and crash carts at a location licensed as a terminal distributor of dangerous drugs.
(a) Notwithstanding the definition of direct supervision in rule 4729:3-1-01 of the Administrative Code, a certified pharmacy technician may stock automated drug storage systems, crash carts, and floor stock at a location licensed as a terminal distributor of dangerous drugs if a pharmacist is not physically present at the licensed location and all of the following apply:
(i) A pharmacist is readily available to answer questions of the certified pharmacy technician;
(ii) A pharmacist is responsible for conducting routine verifications of the activities of the certified pharmacy technician to prevent the diversion of dangerous drugs;
(iii) A pharmacist is fully responsible for all activities conducted by the certified pharmacy technician at the licensed location.
(12) Requesting refill authorizations for dangerous drugs from a prescriber or prescriber’s agent, so long as there is no change from the original prescription;
(13) Accepting new verbal prescription orders, including refill authorizations, for non-controlled drugs from a prescriber or a prescriber’s agent pursuant to all of the following:
(a) The pharmacist on duty who is supervising the activity of the certified pharmacy technician will determine if the technician is competent to receive a verbal order.
(b) The pharmacist on duty who is supervising the activity of the certified pharmacy technician is responsible for the accuracy of a prescription order received by a technician.
(c) The pharmacist on duty must be immediately available to answer questions or discuss the prescription order received by a certified pharmacy technician.
(d) The certified pharmacy technician may not receive a prescription order for a controlled substance.
(e) If applicable, the certified pharmacy technician receiving a prescription order must document the full name of the prescriber’s agent.
(f) The receiving certified pharmacy technician shall immediately reduce the prescription order to writing, which may include entering the information directly into a computerized record keeping system, and shall review the prescription with the pharmacist on duty.
(g) Prior to dispensing, positive identification of the receiving certified pharmacy technician and the pharmacist on duty shall be recorded to identify the responsibility for the receipt of the prescription.
(h) The certified pharmacy technician and the pharmacist on duty must meet all other applicable rules for the receipt of new verbal prescription orders pursuant to agency 4729 of the Administrative Code.
(14) Send or receive copies of non-controlled prescriptions pursuant to all of the following:
(a) The pharmacist on duty who is supervising the activity of the certified pharmacy technician will determine if the technician is competent to send or receive a prescription copy.
(b) The pharmacist on duty who is supervising the activity of the certified pharmacy technician is responsible for the accuracy of a prescription copy that is sent or received by a technician.
(c) The pharmacist on duty must be immediately available to answer questions or discuss the prescription copy that is sent or received by a certified pharmacy technician.
(d) The certified pharmacy technician may not send or receive a prescription copy for a controlled substance.
(e) The pharmacist or certified pharmacy technician receiving a prescription copy from a certified pharmacy technician must document the full names of the sending technician and the technician’s supervising pharmacist. The receiving technician shall immediately reduce the prescription copy to writing, which may include entering the information directly into a computerized record keeping system, and shall review the prescription with the pharmacist on duty. Prior to dispensing, positive identification of the certified pharmacy technician and the pharmacist on duty shall be recorded to identify the responsibility for the receipt of the copy.
(f) The pharmacist or certified pharmacy technician sending a prescription copy to a certified technician must document the full names of the receiving technician and the technician’s supervising pharmacist.
(g) The certified technician and the pharmacist on duty must meet all other applicable rules for the transfer of a prescription copy pursuant agency 4729 of the Administrative Code.
(15) Contacting a prescriber or prescriber’s agent to obtain clarification for a prescription order if the clarification does not require the exercise of professional judgment.
(16) Performing diagnostic laboratory testing pursuant to agency 4729 of the Administrative Code.
(D) A certified pharmacy technician may:
(1) Engage in remote entry of prescriptions in accordance rule 4729:5-5-25 of the Administrative Code; or
(2) Engage in remote entry of medication orders in accordance with rule 4729:5-9-02.15 of the Administrative Code.
(E) In order to perform non-sterile drug compounding, a certified pharmacy technician shall complete the following training requirements prior to compounding non-sterile preparations:
(1) Training shall comply with the requirements set forth in the United States pharmacopeia chapter <795>.
(2) Non-sterile drug compounding training shall be obtained through completion of a site-specific, structured on-the-job didactic and experiential training program and shall not be transferable to another practice site, except between practice sites under common ownership and control.
(3) When the responsible person or a pharmacist designated by the responsible person is satisfied with the employee’s knowledge and proficiency, the responsible person or the responsible person’s designee will sign the documentation records to show that the employee was appropriately trained in accordance with this rule.
(4) Ensuring certified pharmacy technicians are properly trained shall be the responsibility of the terminal distributor of dangerous drugs and the licensee’s responsible person.
(5) All training requirements set forth in this paragraph shall be appropriately documented and made readily retrievable for immediate inspection by an agent of the state board of pharmacy. Documentation shall be maintained by the terminal distributor of dangerous drugs for a minimum of three years.
(F) In order to perform sterile drug compounding, a certified pharmacy technician shall complete the following training requirements prior to compounding sterile preparations:
(1) Training shall comply with the requirements set forth in the United States pharmacopeia chapter <797>.
(2) Sterile drug compounding training shall be obtained through completion of a site-specific, structured on-the-job didactic and experiential training program and shall not be transferable to another practice site, except between practice sites under common ownership and control.
(3) When the responsible person or a pharmacist designated by the responsible person is satisfied with the employee’s knowledge and proficiency, the responsible person or the responsible person’s designee will sign the documentation records to show that the employee was appropriately trained in accordance with this rule.
(4) Ensuring certified pharmacy technicians are properly trained shall be the responsibility of the terminal distributor of dangerous drugs and the licensee’s responsible person.
(5) All training requirements set forth in this paragraph shall be appropriately documented and made readily retrievable for immediate inspection by an agent of the state board of pharmacy. Documentation shall be maintained by the terminal distributor of dangerous drugs for a minimum of three years.
(G) A terminal distributor of dangerous drugs and the licensee’s responsible person shall be responsible for the implementation of policies and procedures for additional training appropriate to duties and responsibilities performed by a certified pharmacy technician as well as an ongoing quality assurance plan to ensure competency.” “Rule 4729:3-3-05 | Certified pharmacy technician administration of diagnostic tests.
(A) A certified pharmacy technician under the direct supervision of a pharmacist may administer clinical laboratory improvement amendments (CLIA) waived diagnostic laboratory testing provided the following conditions are met:
(1) The pharmacy or facility licensed as a terminal distributor of dangerous drugs is certified by the United States department of health and human services (HHS), as a clinical laboratory through the CLIA;
(2) The pharmacy or facility licensed as a terminal distributor of dangerous drugs has obtained a CLIA certificate of waiver from HHS; and
(3) The responsible person of the terminal distributor of dangerous drugs and the terminal distributor of dangerous drugs shall ensure and document that all certified pharmacy technicians conducting CLIA waived tests pursuant to this rule receive appropriate training to conduct testing in a safe and effective manner.
(B) This rule applies only to the administration and evaluation of laboratory testing by individuals licensed or registered in accordance with Chapter 4729. of the Revised Code.
Rule 4729:3-3-06 | Immunization administration.
(A) A certified or registered pharmacy technician who meets the requirements of paragraph (B) of this rule and is working under the direct supervision of a pharmacist who meets the requirements of rule 4729:1-3-02 of the Administrative Code, may administer to an individual who is five years of age or older an immunization for any disease, including an immunization for influenza or COVID-19.
(B) For a certified or registered pharmacy technician to be authorized to engage in the administration of immunizations, the technician shall comply with all the following requirements:
(1) Complete a practical training program that meets the requirements set forth in paragraph (C) of this rule.
(2) Administer immunizations authorized by a prescriber-established protocol that meets the requirements of rule 4729:1-3-02 of the Administrative Code.
(3) Be authorized by the supervising pharmacist to administer immunizations. The pharmacist supervising the technician may prohibit, limit, or restrict the type of immunizations administered, including the age of the patient, by the technician.
(4) Receive and maintain certification to perform basic life-support procedures by successfully completing a basic life-support training course certified by the American red cross, American heart association, American safety and health institute, or other training course approved by the board. Certification shall be obtained and maintained through courses that are conducted in- person or, at a minimum, offer an in-person or electronic hands-on training component.
(5) The pharmacist on duty who is supervising the technician shall be on-site to administer epinephrine or diphenhydramine, or both, to individuals in emergency situations resulting from adverse reactions to the immunizations administered by the registered or certified pharmacy technician.
(6) The pharmacist on duty who is supervising the technician determines if the technician is competent to administer immunizations.
(C) A course in the administration of immunizations developed pursuant paragraph (B) of this rule shall meet the following requirements:
(1) The instructor shall be a licensed health care professional and have the appropriate education and experience to teach a course in the administration of immunizations.
(2) The content must meet the standards established for such courses by the centers for disease control and prevention in the public health service of the United States department of health and human services.
(3) The course shall be conducted by an accreditation council for pharmacy education (ACPE) accredited provider.
(4) The course must be a minimum of six hours in length and include, at a minimum, the following topic areas:
(a) A review of immunology that includes a discussion of the body’s immune system reaction to immunizations.
(b) A review of each immunization recommended by the advisory committee on immunization practices of the centers for disease control and prevention in the United States department of health and human services (6/28/2024):
(i) Disease states associated with the immunization;
(ii) Type or nature of activity of the immunization;
(iii) Administration schedules;
(iv) Routes of administration;
(v) Injection sites;
(vi) Dosages;
(vii) Monitoring and treatment of the patient for adverse reactions;
(viii) Patient populations;
(ix) Precautions and contraindications; and
(x) Proper storage requirements for the immunization.
(c) A review of sterile technique in injectable dosage preparation and administration.
(d) A minimum of one hour of instruction and physical participation in administration techniques.
(e) A review of the proper disposal procedures for contaminated needles and immunizations.
(f) A review of the proper procedures for accidental needle sticks.
(5) The course must provide a method to evaluate the successful comprehension of the content.
(6) The course must provide a method to demonstrate the participant has successfully completed the course.
(D) Courses on immunization administration may be reviewed by the state board of pharmacy. A training course that fails to comply with the requirements set forth in this rule shall be considered in violation of this rule.
(E) The pharmacy employing the technician shall ensure informed consent is obtained pursuant to rule 4729:5-5-04 of the Administrative Code prior to the administration of an immunization.
(F) The pharmacy employing the technician shall ensure the technician maintains the competency and skills necessary to safely administer immunizations. The pharmacy shall ensure the technician has initial and annual documented assessment of competency in immunization administration.
(G) Immunization records shall be maintained in accordance with rule 4729:5-5-04 of the Administrative Code.
(H) The pharmacy where a technician is administering immunizations in accordance with this rule shall comply with the vaccine information statement requirements of the National Vaccine Childhood Injury Act, 42 USC Section 300aa-26 (12/14/1993).
(I) For each immunization administered to an individual by a certified or registered pharmacy technician, other than an immunization for influenza administered to an individual eighteen years of age or older, the pharmacy employing the technician shall be responsible for ensuring the notification of the individual’s primary care provider or, if the individual has no primary care provider, the board of health of the health district in which the individual resides or the authority having the duties of a board of health for that district under section 3709.05 of the Revised Code. The notice shall be given not later than thirty days after the immunization is administered. Notification shall be conducted using one of the following methods that is capable of confirming delivery of the required notification:
(1) Electronic mail;
(2) Interoperable electronic medical records system;
(3) Facsimile;
(4) Electronic prescribing system;
(5) Electronic pharmacy record system;
(6) Reporting to the state’s immunization registry;
(7) Documented verbal communication; or
(8) Any other method of notification that might reasonably be expected to allow for the confirmed transmission of the required notification.
(J) For each immunization administered by a certified pharmacy technician or registered pharmacy technician to an individual who is younger than eighteen years of age, the certified pharmacy technician or registered pharmacy technician shall inform the individual’s parent or legal guardian of the importance of well child visits with a pediatrician or other primary care provider and shall refer patients when appropriate.
(K) The pharmacy employing a certified or registered technician authorized to provide immunizations in accordance with this rule, shall maintain, or have immediate access to, the following records on file at the location(s) where the pharmacy technician administers immunizations in accordance with this rule:
(1) Proof of successful completion of a training course specified in paragraph (C) of this rule;
(2) Proof of maintenance of certification to perform basic life-support procedures in accordance with paragraph (B)(4) of this rule; and
(3) Proof of competency assessments as required in paragraph (F) of this rule.
(L) A pharmacist practicing within an outpatient pharmacy shall not supervise more than three pharmacy personnel engaged in the administration of immunizations pursuant to this rule and rule 4729:2-3-03 of the Administrative Code.
(M) A pharmacist supervising an immunization clinic outside of an outpatient pharmacy shall not supervise more than six pharmacy personnel engaged in the administration of immunizations pursuant to this rule and rule 4729:2-3-03 of the Administrative Code.” “Rule 4729:3-5-02 | Continuing Education Requirements for Registered and Certified Technicians.
(A) As a condition for the renewal of a registration as a registered pharmacy technician, the registrant shall complete a total of ten contact hours (1.0 C.E.Us) of continuing pharmacy education during the twenty-four months preceding the expiration date of the technician’s registration. The continuing pharmacy education shall be in pharmacy technician-specific subject matter and shall include, at a minimum, the following:
(1) Two contact hours (0.2 C.E.Us) of continuing pharmacy education shall be in the subject of pharmacy jurisprudence (law).
(2) Two contact hours (0.2 C.E.Us) of continuing pharmacy education shall be in the subject of patient or medication safety.
(B) Paragraph (A) of this rule does not apply to registered pharmacy technicians that obtain an initial registration within six months of the expiration date of the registration.
(C) A registered pharmacy technician may satisfy up to one-third of the technician’s continuing education requirements by providing health care services as a volunteer in accordance with section 4745.04 of the Revised Code. The location where health care services are provided shall be an approved in-state provider of volunteer healthcare services in accordance with agency 4729 of the Administrative Code.
(D) Registered pharmacy technicians shall keep all certificates and other documented evidence of participation which have been issued by a non-A.C.P.E. accredited provider for which the pharmacy technician has claimed continuing education units towards renewal of the technician’s registration for a period of one year following the year in which evidence was required for renewal.
(1) Documentation, as determined by the state board of pharmacy, shall be submitted only when requested by the board.
(2) The board shall monitor compliance by conducting an audit of registrants.
(3) The board shall require the reporting of continuing education units to a national and/or state register.
(E) As a condition for the renewal of a registration as a certified pharmacy technician, the registrant shall complete all continuing education requirements necessary to maintain the registrant’s pharmacy technician certification from an organization that has been recognized by the board.
The data contained in this 2012 Annual Scorecard are accurate as of December 2012 . Because statutes and regulations are continually revised, the data are subject to change. These data have been verified with the state board of pharmacy. This scorecard is updated on an annual basis in order to incorporate statutory and regulatory changes. A new scorecard will be issued in July 2013.
Scoring rationale for Education and Training:
In order to protect the public and help ensure patient safety, it is important that pharmacy technicians are properly educated and trained. The most rigorous training is accredited training. The sole entity empowered to accredit pharmacy-technician training programs is the American Society of Health-System Pharmacists (ASHP). Please note that this is “programmatic accreditation” – not “institutional accreditation.” It is the content of the training program – as measured against established standards – that is being evaluated and accredited. Accredited training is vital to protecting patient safety because it means that a pharmacy-technician training program has met established quality standards to provide assurance and confidence to the public. For more information, please see http://www.ashp.org/menu/Accreditation/TechnicianAccreditation.aspx.
Scoring rationale for Certification:
Certification is the process by which a nongovernmental agency or association grants recognition to an individual who has met certain predetermined qualifications specified by that agency or association. This is often determined by an examination process. Numerous organizations have recommended that the certification exam conducted by the Pharmacy Technician Certification Board (PTCB) should be recognized as the sole, nationally-accredited certification exam for pharmacy technician certification – including the National Association of Boards of Pharmacy (NABP), the Texas State Board of Pharmacy (TSBP), and the U.S. Department of Veterans Affairs (VA). In a recent report, NABP recommended that states be encouraged to “recognize certification by the Pharmacy Technician Certification Board (PTCB).” Moreover, NABP performed a psychometric audit of the PTCB’s pharmacy technician certification examination (PTCE) in 2001 and determined that the PTCE is psychometrically sound, defensible, and valid. In May 2010, the TSBP awarded the PTCB with the Pharmacy Technician Certification Provider contract in Texas. PTCB was selected for the contract after a rigorous bidding and evaluation process that included formal reviews and evaluations from three independent psychometricians. TSBP confidently recognizes PTCB as the single provider of certification examinations for pharmacy technicians. In addition, in June 2010, the VA began requiring PTCB certification for VA pharmacy technicians employed at grade GS-6 and above.
Scoring rationale for Registration/Licensure:
Registration/licensure is the process by which the state maintains a list of all pharmacy technicians in the state and grants permission for an individual to work as a pharmacy technician in the state based on the applicant’s completion of all pre-requisites to registration/licensure – such as required training and certification.
Scoring rationale for Continuing Education:
Continuing education enables pharmacy technicians to fulfill their professional responsibility to maintain competence and up-to-date knowledge and skills in an environment of technological advances and increasingly complex, new medications and therapies.
Our Mission
The Emily Jerry Foundation is determined to help make our nation’s, world renowned, medical facilities safer for everyone, beginning with our babies and children. We are accomplishing this very important objective by focusing on increasing public awareness of key patient safety related issues and identifying technology and best practices that are proven to minimize the “human error” component of medicine. Through our ongoing efforts The Emily Jerry Foundation is working hard to save lives every day.
Recent Posts
Archives