
Keynote at Mount Sinai Health System’s Medication Safety Together Summit
November 24, 2025
By ejfadmin
I was truly honored to represent the Emily Jerry Foundation last week at Mount Sinai Health System, where I had the privilege of delivering the keynote address to kick off their Medication Safety Together – An Interdisciplinary Summit… Read More



Arkansas Scorecard
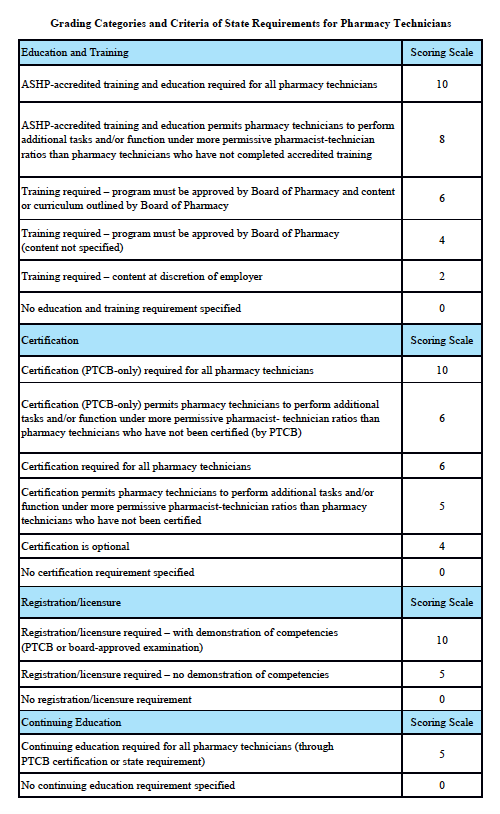
Grading Scale:
A – 85-100%, B – 70-84.9%, C – 55-69.9%, D – 40-54.9%, F – 0-39.9%
Grading Categories & Criteria
Arkansas Law
I. Laws
https://healthy.arkansas.gov/wp-content/uploads/Lawbook-2025-May-9.pdf
17-92-101. Definitions.
(C)
(i) The Arkansas State Board of Pharmacy may permit pharmacy technicians other than pharmacists or interns to perform some or all of those functions described in Arkansas State Board of Pharmacy rules under the direct, personal supervision of a licensed pharmacist under rules defining the minimum qualifications of such employees, the ratio of pharmacy technicians to supervising pharmacists, and the scope of the duties, practices, and procedures that the Arkansas State Board of Pharmacy determines will promote the delivery of competent, professional pharmaceutical services and promote the public health and welfare.
(ii) A pharmacy technician may administer vaccines and immunizations to a person three (3) years of age or older if delegated to do so by a supervising pharmacist, but may not administer
other medications.
(iii) The conduct of a pharmacy technician is the responsibility of the pharmacist-in-charge and supervising pharmacist of the pharmacy who shall not permit the employee to perform any act, task, or function that involves the exercise of independent judgment by the employee.
(iv) Pharmacy products prepared by pharmacy technicians shall be verified for accuracy by the supervising pharmacist before release for patient use, and the verification shall be documented.
(v) The use of pharmacy technicians in a manner not authorized by this chapter or rules promulgated hereunder shall be unprofessional conduct by the pharmacist-in-charge and the supervising pharmacist.
(vi) It is recognized that hospital pharmacy technicians as defined in § 17-92-602(4) are governed by the Hospital Pharmacies Act, § 17-92-601 et seq., and related Arkansas State Board of Pharmacy rules developed under the Hospital Pharmacies Act, § 17-92-601 et seq.;” “17-92-301. License required
(a) No person shall perform any of the acts constituting the practice of pharmacy unless the person is:
(1) A licensed pharmacist;
(2) A student or graduate of a recognized college of pharmacy serving an internship under an internship program established and regulated by the Arkansas State Board of Pharmacy;
(3) A pharmacy technician performing the limited functions permitted under this chapter and rules promulgated hereunder; or
(4) A hospital pharmacy technician as defined in § 17-92-602 performing the limited functions permitted under that subchapter and rules promulgated thereunder.
(b) No person other than a licensed pharmacist shall use the term “doctor of pharmacy” or “Pharm.D”.” “17-92-310. Failure to renew. (no other changes to the content)
(a)
(1)
(A) All retail pharmacy permits, out-of-state pharmacy permits, specialty pharmacy permits, and pharmacist licenses shall expire on December 31 of the first odd-numbered year following the date
of issuance.
(B) All preceptor permits shall expire on December 31 of the first odd-numbered year following the date of issuance.
(C)
(i)
(a) Intern licenses issued to foreign graduates shall expire on December 31 of the second calendar year following the date of issuance.
(b) However, an intern license issued to a foreign graduate shall expire when the intern is issued a pharmacist license.
(ii)
(a) An intern license issued to a student intern shall remain valid as long as the intern maintains active student status in a college of pharmacy approved by the Arkansas State Board of Pharmacy and for six (6) months following graduation.
(b) An intern license issued to a student intern shall expire six (6) months following graduation.
(c) An intern license issued to a student intern may be reinstated if the intern resumes active student status in a board-approved college of pharmacy and applies for reinstatement.
(d) An intern license issued to a student intern shall expire when the intern is issued a pharmacist license.
(D) All pharmacy technician permits, hospital pharmacy permits, ambulatory care center pharmaceutical services permits, wholesale distributors, third-party logistics providers, manufacturers, or outsourcing facilities of legend or controlled substance permits, wholesale distributors of medical equipment, legend devices, and medical gases permits, institutional pharmaceutical services permits, List I chemical permits, and any other permit, license, registration, or certificate issued by the board and not covered in subdivisions (a)(1)(A)-(C) of this section shall expire on December 31 of the first even-numbered year following the date of the issuance of the permit, license, registration, or certificate.
(2) Every license, permit, registration, and certificate not renewed within ninety (90) days after expiration thereof shall be void.
(b) The penalty for late payment of renewal for pharmacists, pharmacies, wholesaler/manufacturer of legend drugs and controlled substances, hospital, and institutional permits shall be as listed in § 17-92-108, and if renewal remains unpaid by April 1 of the year, the license shall be void.
(c) If a pharmacist’s license is not renewed by April 1, the fee for reinstatement shall be as stated in § 17-92-108.
(d) If a pharmacist’s license has not been renewed for more than two (2) years, the board shall evaluate the former pharmacist to determine his or her continued ability to practice pharmacy safely with regard to the public health and safety, and the board shall establish conditions for the safe reentry into practice of the profession.” “17-92-604. Regulatory authority
(a) The Arkansas State Board of Pharmacy shall adopt, promulgate, and enforce rules and standards as may be necessary to the regulation of the operation of a hospital pharmacy and for the accomplishment of all other purposes of this subchapter.
(b) The board may modify, amend, or rescind the rules and standards, provided the modification, amendment, or rescission does not in any manner defeat the purposes of this subchapter.
Paragraphs (b)(1), (b)(2)(A), (b)(2)(B), (c) are struck.
17-92-801. Powers and duties of Arkansas State Board of Pharmacy.
(a) The Arkansas State Board of Pharmacy shall provide that hospital pharmacy technicians as in § 17-92-602 and pharmacy technicians as in § 17-92-101(18)(C), and hereinafter referred to as pharmacy technicians, register with or be certified by the board, or both.
(b) The board may provide reasonable qualifications for a person to be certified as a pharmacy technician or registered as a pharmacy technician, or both, including, without limitation, the education, training, and testing that the board deems necessary to preserve and protect the public health.
(c) The board may suspend or revoke the registration of any person certified as a pharmacy technician or registered as a pharmacy technician, or both, but only after an opportunity for a hearing before the board upon reasonable notice to the person in writing.
(d) Grounds for suspension or revocation of registration or certification as a pharmacy technician, or both, are the following:
(1) Violation of any law or rule regarding the practice of pharmacy;
(2) Violation of any law or rule regarding legend drugs or controlled substances; or
(3) Violation of any rule adopted by the board regarding pharmacy technicians” Continuing education link 17 CAR S 160.803 Implementation of pharmacy continuing education Subpart 9. Pharmacy Technicians – Registration/Permit Required has no content in it.
17 CAR § 160-902. Registration required.
(a) A pharmacy technician shall:
(1) Register with the Arkansas State Board of Pharmacy on a form provided by the board; and
(2) Undergo a criminal background check pursuant to Subpart 31.
(b) The registration shall expire on December 31 biennially as provided in 17 CAR § 160-107.
(c) The registration fee for a pharmacy technician shall be defined in 17 CAR § 160-107.
(d)(1) No person shall work as a pharmacy technician prior to the board issuing a certificate of registration and a permit.
(2) The permit shall be prominently displayed for public perusal in any pharmacy where the technician is working.
(3) The pharmacist-in-charge shall determine that the person is registered as a pharmacy technician and that the board has issued a permit for the technician before the technician performs any tasks identified in 17 CAR § 160-905 or 17 CAR § 160-906.
(e) If there is a change of mailing address for the pharmacy technician, the pharmacy technician shall immediately notify the board, in writing, of the new address.
(f) When a pharmacy technician leaves the employment of a pharmacy, the pharmacist-in-charge shall notify the board, in writing, within fourteen (14) days.
(g)(1) Any concurrent or subsequent employment at any pharmacy shall be reported to the board by both the pharmacy technician and the pharmacist-in-charge of the pharmacy where the pharmacy technician will be working.
(2) The pharmacist-in-charge must notify the board, in writing, of the exact date when the pharmacy technician will begin working.
(3) The pharmacy technician shall not work at that location until the board has received said notification.
(h) A pharmacy technician shall identify himself or herself as such in any telephone conversation regarding the functions of a pharmacy technician while on duty in the pharmacy.
(i)(1) If the pharmacy technician is suspected to have, or evidence exists that a pharmacy technician may have violated any law or rule regarding the practice of pharmacy, legend drugs, or controlled substances, the pharmacist-in-charge shall notify the board in writing:
(A) Within ten (10) days; or
(B) Immediately if any danger to the public health or safety may exist.
(2) Any other pharmacist, whether or not practicing in the same pharmacy, who has such knowledge or suspicion shall notify the board in a like manner.
(j)(1) The board may, after notice and hearing, suspend or revoke the permit of a pharmacy technician upon a finding of the following:
(A) Violation of this part;
(B) Violation of any law or rule regarding the practice of pharmacy; or
(C) Violation of any law or rule related to legend drugs or controlled substances.
(2) The board shall follow the same procedures for hearings for pharmacy technicians as applicable to hearings for pharmacists as set forth in Arkansas Code § 17-92-101 et seq. and board rules.” ” Recommend adding:
17 CAR § 160-903. A pharmacy technician shall.
A pharmacy technician shall:
(1) Conduct himself or herself professionally in conformity with all applicable federal, state, and municipal laws and rules in his or her relationship with:
(A) The public;
(B) Healthcare professionals; and
(C) Pharmacists;
(2) Hold to the strictest confidences all knowledge concerning patrons, their prescriptions, and other confidences entrusted or acquired by him or her, divulging in the interest of the patron only:
(A) By proper release forms; or
(B) Where required for proper compliance with legal authority; and
(3) Provide valid and sufficient checks in payment for licenses or renewals.
17 CAR § 160-904. Qualifications.
(a) A high school graduate or a recognized graduate equivalency degree (GED).
(b)(1) The applicant must complete a criminal background check pursuant to Subpart 31.
(2) If the pharmacy technician has a past record of alcohol or drug addiction or past record of violation of any law related to controlled substances, registration must be prior-approved by the Arkansas State Board of Pharmacy.
17 CAR § 160-905. Tasks, responsibilities, and duties of the pharmacy technician.
(a)(1) A pharmacy technician may assist the pharmacist in performing the following specific tasks in accordance with specific written policy and procedures established by the pharmacist-in-charge covering the areas described in this section.
(2) The supervising pharmacist is responsible for all tasks performed by the pharmacy technician.
(3) All tasks performed by the pharmacy technician must be supervised, checked, and approved by the supervising pharmacist.
(4) If the pharmacy technician performs any other task that is defined as the practice of pharmacy, it will be considered a violation.
(b) Approved tasks:
(1)(A) Placing, packing, pouring, or putting in a container for dispensing, sale, distribution, transferring possession of, vending, or bartering any drug, medicine, poison, or chemical that, under the laws of the United States or the State of Arkansas, may be sold or dispensed only on the prescription of a practitioner authorized by law to prescribe:
(i) Drugs;
(ii) Medicines;
(iii) Poisons; or
(iv) Chemicals.
(B) This shall also include the adding of water for reconstitution of oral antibiotic liquids;
(2) Placing in or affixing upon any container described in this part a label required to be placed upon drugs, medicines, poisons, or chemicals sold or dispensed upon prescription of a practitioner authorized by law to prescribe those:
(A) Drugs;
(B) Medicines;
(C) Poisons; or
(D) Chemicals;
(3) Selecting, taking from, and replacing upon shelves in the prescription department of a pharmacy or apothecary drugs, medicines, chemicals, or poisons that are required by the law of the United States or the State of Arkansas to be sold or dispensed only on prescription of a practitioner authorized by law to prescribe them;
(4)(A) In a manual system, preparing, typing, or writing labels to be placed or affixed on any container described in Arkansas Code § 17-92-101 on which a label is required to be placed upon drugs, medicines, poisons, or chemicals sold or dispensed upon prescription of a practitioner authorized by law to prescribe those:
(i) Drugs;
(ii) Medicines;
(iii) Poisons; or
(iv) Chemicals.
(B)(i) In a computer system, a pharmacy technician may enter information into the pharmacy computer.
(ii) The pharmacy technician shall not make any judgment decisions that could affect patient care.
(iii) The final verification of prescription information entered into the computer shall be made by the supervising pharmacist prior to dispensing, who is then totally responsible for all aspects of the data and data entry;
(5)(A) A pharmacy technician may obtain prescriber authorization for prescription refills provided that nothing about the prescription is changed.
(B) A pharmacy technician shall not receive prescriber authorization for a new prescription by telephone or by other verbal communication;
(6)(A) Prepackaging and labeling of multi-dose and unit dose packages of medication.
(B) The pharmacist must:
(i) Establish the procedures, including selection of:
(a) Containers;
(b) Labels; and
(c) Lot numbers; and
(ii) Check the finished task;
(7) Dose-picking for unit dose cart fill for a hospital or for a nursing home patient;
(8)(A) Nursing unit checks in a hospital or nursing home.
(B) Pharmacy technicians may check nursing units for proper medication storage and other related floor stock medication issues.
(C) Any related medication storage problems or concerns shall be documented and initialed by a pharmacist;
(9) Patient and medication records. The recording of patient or medication information in a manual or electronic system for later validation by the pharmacist may be performed by pharmacy technicians; and
(10) The pharmacy technician shall not make any judgment decisions that could affect patient care.
(c)(1) A pharmacy technician may assist in the following tasks when the pharmacist-in-charge has established a specific written policy and procedure for reconstitution of prefabricated noninjectable medication, bulk compounding, and/or preparation of parenteral products that establishes the:
(A) Order of addition of ingredients; and
(B) Point at which the ingredients will be checked by the pharmacist, and the point at which the final product will be checked for:
(i) Integrity;
(ii) Correctness; and
(iii) Pharmaceutical elegance.
(2)(A) Prior to any of these tasks being carried out by a pharmacy technician:
(i) The technician shall successfully complete an initial training, assessment of skills program, and test pursuant to a written training and assessment procedure established by the pharmacist-in-charge as provided in 17 CAR § 160-906; and
(ii) The pharmacist supervising a technician who engages in the above-referenced reconstitution, bulk compounding, and/or preparation of parenteral product shall:
(a) Perform all calculations of ingredients; and
(b) Provide written directions for measurement of ingredients by the technician.
(B) Prior to dispensing any of said products for administration, the supervising pharmacist shall verify and approve in written form all ingredients as well as the final product.
(d)(1) Bulk reconstitution of prefabricated noninjectable medication may include addition of multiple additives.
(2) Bulk compounding may include such items as sterile bulk solutions for small volume injectables, sterile irrigating solutions, products prepared in relatively large volume for internal or external use by patients, and reagents or other products for the pharmacy or other departments of the facility.
(3) Preparation of parenteral products.
(4) Pharmacy technicians may:
(A) Reconstitute and withdraw any amount, i.e., partial or entire amount, of an injectable medication to be administered to a patient; and
(B) Reconstitute, withdraw, and add any amount, i.e., partial or entire amount, of one (1) or more injectable products to an IV solution to be administered to a patient.
17 CAR § 160-1009. Responsibility of pharmacist, intern, or pharmacy technician.
(a) Any pharmacist, intern, or pharmacy technician participating in the preparation of orders or dispensing of prescriptions and/or any pharmacist who is responsible for supervising pharmacy personnel participating in the preparation of orders or dispensing of prescriptions is responsible for the validity and legality of the order or prescription.
(b) Any pharmacist who is responsible for supervising pharmacy personnel is also responsible for any shortage of drugs classified as controlled drugs under state or federal law that occurs under their supervision.
(c) In a pharmacy’s electronic data processing system that can delineate the individual steps in the prescription filling process, the pharmacist overseeing each step would be specifically responsible for that part of the process.
(d) In a pharmacy’s electronic data processing system that is not capable of delineating the individual steps in the prescription filling process, the pharmacist or pharmacists involved in the process will share a corresponding liability for each prescription filled.
References
Pharmacy Practice Act
http://pharmacyboard.arkansas.gov/licenseeInfo/Documents/lawBook/pharmacyPracticeAct.pdf
Regulation 3
http://pharmacyboard.arkansas.gov/licenseeInfo/Documents/lawBook/regulation03Nov03.pdf
The data contained in this 2012 Annual Scorecard are accurate as of December 2012 . Because statutes and regulations are continually revised, the data are subject to change. These data have been verified with the state board of pharmacy. This scorecard is updated on an annual basis in order to incorporate statutory and regulatory changes. A new scorecard will be issued in July 2013.
Scoring rationale for Education and Training:
In order to protect the public and help ensure patient safety, it is important that pharmacy technicians are properly educated and trained. The most rigorous training is accredited training. The sole entity empowered to accredit pharmacy-technician training programs is the American Society of Health-System Pharmacists (ASHP). Please note that this is “programmatic accreditation” – not “institutional accreditation.” It is the content of the training program – as measured against established standards – that is being evaluated and accredited. Accredited training is vital to protecting patient safety because it means that a pharmacy-technician training program has met established quality standards to provide assurance and confidence to the public. For more information, please see http://www.ashp.org/menu/Accreditation/TechnicianAccreditation.aspx.
Scoring rationale for Certification:
Certification is the process by which a nongovernmental agency or association grants recognition to an individual who has met certain predetermined qualifications specified by that agency or association. This is often determined by an examination process. Numerous organizations have recommended that the certification exam conducted by the Pharmacy Technician Certification Board (PTCB) should be recognized as the sole, nationally-accredited certification exam for pharmacy technician certification – including the National Association of Boards of Pharmacy (NABP), the Texas State Board of Pharmacy (TSBP), and the U.S. Department of Veterans Affairs (VA). In a recent report, NABP recommended that states be encouraged to “recognize certification by the Pharmacy Technician Certification Board (PTCB).” Moreover, NABP performed a psychometric audit of the PTCB’s pharmacy technician certification examination (PTCE) in 2001 and determined that the PTCE is psychometrically sound, defensible, and valid. In May 2010, the TSBP awarded the PTCB with the Pharmacy Technician Certification Provider contract in Texas. PTCB was selected for the contract after a rigorous bidding and evaluation process that included formal reviews and evaluations from three independent psychometricians. TSBP confidently recognizes PTCB as the single provider of certification examinations for pharmacy technicians. In addition, in June 2010, the VA began requiring PTCB certification for VA pharmacy technicians employed at grade GS-6 and above.
Scoring rationale for Registration/Licensure:
Registration/licensure is the process by which the state maintains a list of all pharmacy technicians in the state and grants permission for an individual to work as a pharmacy technician in the state based on the applicant’s completion of all pre-requisites to registration/licensure – such as required training and certification.
Scoring rationale for Continuing Education:
Continuing education enables pharmacy technicians to fulfill their professional responsibility to maintain competence and up-to-date knowledge and skills in an environment of technological advances and increasingly complex, new medications and therapies.
Our Mission
The Emily Jerry Foundation is determined to help make our nation’s, world renowned, medical facilities safer for everyone, beginning with our babies and children. We are accomplishing this very important objective by focusing on increasing public awareness of key patient safety related issues and identifying technology and best practices that are proven to minimize the “human error” component of medicine. Through our ongoing efforts The Emily Jerry Foundation is working hard to save lives every day.
Recent Posts
Archives