
Keynote at Mount Sinai Health System’s Medication Safety Together Summit
November 24, 2025
By ejfadmin
I was truly honored to represent the Emily Jerry Foundation last week at Mount Sinai Health System, where I had the privilege of delivering the keynote address to kick off their Medication Safety Together – An Interdisciplinary Summit… Read More



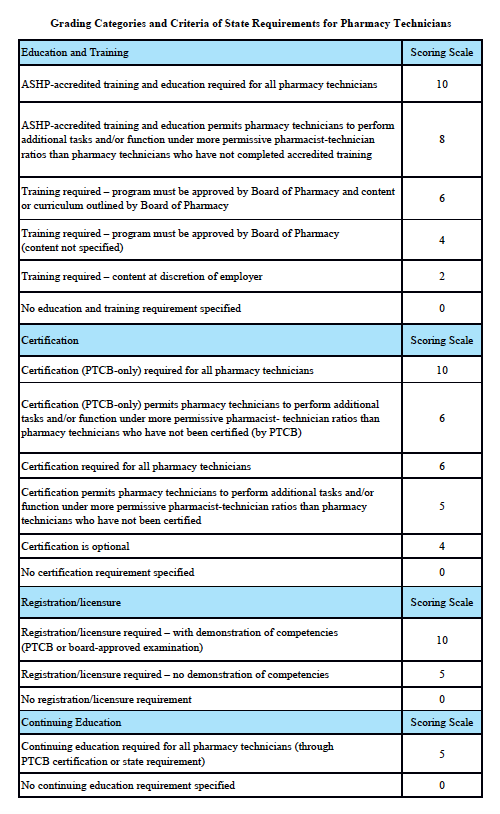
North Dakota Scorecard
Grading Scale:
A – 85-100%, B – 70-84.9%, C – 55-69.9%, D – 40-54.9%, F – 0-39.9%
Grading Categories & Criteria
North Dakota Law
I. LAWS
http://www.legis.nd.gov/cencode/t43c15.pdf
43-15-01. Definitions.
23. “”Pharmacy technician”” means a person registered by the board who is employed by a
pharmacy to assist licensed pharmacists in the practice of pharmacy by performing
specific tasks delegated by and under the immediate personal supervision and control
of a licensed pharmacist, as permitted by the board.” “43-15-10. Powers of board.
In addition to other powers provided by law, the board shall have the following powers and duties, which shall be exercised in conformity with chapter 28-32 in order to protect the public health, welfare, and safety:
1. To place on probation, reprimand, or fine any pharmacy, pharmacist, or pharmacy intern or pharmacy technician; or refuse to issue or renew, or suspend, revoke, restrict, or cancel, the license, permit, or registration of any pharmacy, pharmacist, or pharmacy intern or pharmacy technician, if any of the following grounds apply and the pharmacy, pharmacist, or pharmacy intern or pharmacy technician:
a. Is addicted to any alcohol or drug habit.
b. Uses any advertising statements of a character tending to deceive or mislead the public.
c. Is subject to drug or alcohol dependency or abuse.
d. Permits or engages in the unauthorized sale of narcotic drugs or controlled substances.
e. Permits or engages an unauthorized person to practice pharmacy.
f. Is mentally or physically incompetent to handle pharmaceutical duties.
g. Is guilty of fraud, deception, or misrepresentation in passing the pharmacist examination.
h. Is found by the board in violation of any of the provisions of the laws regulating drugs, pharmacies, and pharmacists or interns and technicians or the rules and regulations established by the board.
i. Is found to have engaged in unprofessional conduct as that term is defined by the rules of the board.
j. Is subject to incapacity of a nature that prevents a pharmacist from engaging in the practice of pharmacy with reasonable skill, competence, and safety to the public.
k. Is found guilty by a court of competent jurisdiction of one or more of the following:
(1) A felony, as defined by the statutes of North Dakota.
(2) Any act involving moral turpitude or gross immorality.
(3) Violations of the pharmacy or the drug laws of North Dakota or rules and regulations pertaining thereto, or of statutes, rules or regulations of any other state, or of the federal government.
l. Commits fraud or intentional misrepresentation in securing the issuance or renewal of a license or pharmacy permit.
m. Sells, dispenses, or compounds any drug while on duty and while under the influence of alcohol or while under the influence of a controlled substance without a practitioner’s prescription.
n. Discloses confidential information to any person, except as authorized by law.
2. To prescribe rules and regulations not inconsistent with this chapter governing the cancellation or suspension of a license.
3. To examine and license as pharmacist any applicant found entitled to such license.
4. To prescribe rules and regulations for the guidance of its members, officers, and employees, and to ensure the proper and orderly dispatch of its business.
5. To employ and pay such persons as it may deem necessary to inspect pharmacies in this state, investigate pharmacies for the information of the board, procure evidence in any proceeding pending before the board, or procure evidence in aid of any
prosecution or action in any court commenced or about to be commenced by or against the board in relation to any matter in which the board has any duty to perform.
6. To employ and pay counsel to advise the board or to prosecute or defend any action or proceeding commenced by or against the board or pending before it.
7. To grant permits and renewals thereof for the establishment and operation of pharmacies.
8. Only for good cause to cancel, revoke, or suspend permits and renewals thereof for the establishment and operation of pharmacies.
9. To prescribe reasonable and nondiscriminatory rules and regulations in regard to granting, renewing, canceling, revoking, or suspending permits and renewals for establishing and operating pharmacies.
10. Action by the board canceling, revoking, suspending, or refusing to renew a permit to establish or operate a pharmacy shall not be enforced for thirty days after notice has been given an aggrieved party by the board, nor during the time that an appeal by such aggrieved party is pending and until such appeal is finally determined.
11. To prescribe reasonable rules and regulations relating to the physical design of space occupied by a pharmacy to ensure appropriate control of and safeguards over the contents of such pharmacy.
12. To regulate and control the practice of pharmacy in North Dakota.
13. To adopt, amend, and repeal rules for the regulation of pharmacies and pharmacists providing radiopharmaceutical services, including special training, education, and experience for pharmacists and physical design of space, safeguards, and equipment for pharmacies.
14. To adopt, amend, and repeal rules determined necessary by the board for the proper administration and enforcement of this chapter, chapter 19-02.1 as that chapter pertains to drugs, subject to approval of the commissioner of the department of health and human services or designee, and chapter 19-03.1.
15. The board or its authorized representatives may investigate and gather evidence concerning alleged violations of the provisions of chapter 43-15, chapter 19-02.1 that pertains to drugs, chapters 19-03.1, 19-03.2, and 19-04, or of the rules of the board. Board investigative files are confidential and may not be considered public records or open records for purposes of section 44-04-18, until a complaint is filed or a decision made by the board not to file a complaint.
16. In addition to other remedies, the board may apply to the district court in the jurisdiction of an alleged violation, and that court has jurisdiction upon hearing and for cause shown, to grant a temporary or permanent injunction restraining any person from violating any provision of chapter 43-15, chapter 19-02.1 pertaining to drugs, and
chapter 19-03.1, whether or not there exists an adequate remedy at law. Whenever a duly authorized representative of the board finds or has probable cause to believe that any drug or device is adulterated, misbranded, mislabeled, or improperly identified, within the meaning of chapter 19-02.1, the representative shall affix to that drug or device a tag or other appropriate marking giving notice that the article is or is suspected of being adulterated, misbranded, mislabeled, or improperly identified, has been detained or embargoed and warning all persons not to remove or dispose of
such article by sale or otherwise until provision for removal or disposal is given by the board or its agents or the court. No person may remove or dispose of such embargoed drug or device by sale or otherwise without the permission of the board or its agent, or,
after summary proceedings have been instituted, without permission from the court.
17. When a drug or device detained or embargoed has been declared by such representative to be adulterated, misbranded, mislabeled, or improperly identified, the board shall, as soon as practical thereafter, petition the district court in whose jurisdiction the article is detained or embargoed for an order for condemnation of such article. If the judge determines that the drug or device so detained or embargoed is not adulterated, misbranded, mislabeled, or improperly identified, the board shall direct the immediate removal of the tag or other marking. If the court finds the detained or embargoed drug or device is adulterated, misbranded, mislabeled, or improperly identified, such drug or device, after entry of the decree, shall be destroyed at the expense of the owner under the supervision of a board representative and all court costs and fees, storage, and other proper expense shall be borne by the owner of such drug or device. When the adulteration, misbranding, mislabeling, or improper identification can be corrected by proper labeling or processing of the drug or device, the court, after entry of the decree and after such costs, fees, and expenses have been paid and a good and sufficient bond has been posted, may direct that such drug or device be delivered to the owner for labeling or processing under the supervision of a board representative. Expense of supervision shall be paid by the owner. Bond posted shall be returned to the owner of the drug or device on representation to the court by the board that the drug or device is no longer in violation of the embargo and the expense of supervision has been paid. Nothing in this section shall be construed to require the board to report violations whenever the board believes the public’s interest will be adequately served in the circumstances by a suitable written notice or warning.
18. The board shall establish a bill of rights for patients concerning the health care services a patient may expect in regard to pharmaceutical care.
19. To adopt, amend, and repeal rules as may be deemed necessary by the board to register pharmacy technicians pursuant to qualifications established by the board, to charge a pharmacy technician an annual registration fee not to exceed fifty dollars, to specify tasks associated with and included in the practice of pharmacy which may be delegated by a licensed pharmacist to a registered pharmacy technician, to provide for suspension or revocation of a pharmacy technician’s registration, and to regulate and control pharmacy technicians. The board may allocate up to fifty percent of the amount of the registration fee to an appropriate pharmacy technician association for its general operating expenses, including pharmacy technician education and development standards.
20. To require the self-reporting by an applicant or a licensee of any information the board determines may indicate possible deficiencies in practice, performance, fitness, or qualifications.
21. To require information regarding an applicant’s or licensee’s fitness, qualifications, and previous professional record and performance from recognized data sources, including the national association of boards of pharmacy data bank, other data repositories, licensing and disciplinary authorities of other jurisdictions, professional education and training institutions, liability insurers, health care institutions, and law enforcement agencies be reported to the board. The board may require an applicant for licensure or a licensee who is the subject of a disciplinary investigation to submit to a statewide and nationwide criminal history record check. The nationwide criminal history record check must be conducted in the manner provided by section 12-60-24. All costs associated with obtaining a background check are the responsibility of the licensee or applicant.
22. To adopt, amend, and repeal rules as may be deemed necessary by the board to register veterinary dispensing technicians pursuant to qualifications established by the board, to charge a veterinary dispensing technician an annual registration fee not to exceed fifty dollars, to provide for suspension or revocation of a veterinary dispensing technician’s registration, to provide for suspension or revocation of a veterinary retail facility’s license, to regulate and control veterinary retail facilities, and to regulate and control veterinary dispensing technicians.
23. To establish limited prescriptive authority for individuals to distribute opioid antagonist kits, also known as “”Naloxone rescue kits””. If the board establishes limited prescriptive authority under this subsection, the board shall adopt rules to establish standards that may include training, certification, and continuing education requirements.
24. To establish limited prescriptive authority through a statewide protocol for public health issues within the scope of practice for a pharmacist. The board shall adopt rules to establish standards of care.” “43-15-14. Unlawful practice of pharmacy.
1. Applicability. No person may engage in the practice of pharmacy unless licensed to practice pharmacy under this chapter, except that a registered pharmacy technician may perform specific tasks delegated by and under the immediate personal supervision and control of a licensed pharmacist, as permitted under rules adopted by the board. Physicians or other practitioners as defined in this chapter who are licensed under the laws of this state may dispense and administer prescription drugs to their patients in the practice of their respective professions if specifically authorized to do so by state law.
2. Penalties. Any person who is found by the board to have unlawfully engaged in the practice of pharmacy is subject to a fine to be imposed by the board not to exceed one thousand dollars for each offense. Each violation of this chapter or the rules adopted under this chapter pertaining to unlawfully engaging in the practice of pharmacy also constitutes a class B misdemeanor.
3. A pharmacy or licensed pharmacist that utilizes the services of a registered pharmacy technician as permitted by the board, may not be considered as aiding and abetting an unauthorized person to practice pharmacy; provided, however, that the pharmacy or licensed pharmacist must retain responsibility for any act performed by a registered pharmacy technician in the course of the registered pharmacy technician’s employment.
North Dakota Administrative Code Title 61
https://ndlegis.gov/prod/agency-rules/north-dakota-administrative-code/
61-02-07.1-02. Definitions.
1. “”Pharmacy technician”” means a person registered by the board of pharmacy who is employed by a pharmacy under the responsibility of the pharmacist-in-charge or a staff pharmacist so designated by the pharmacist-in-charge, to assist in the technical services of preparing
pharmaceuticals for final dispensing by a licensed pharmacist in compliance with subsection 4 of North Dakota Century Code section 43-15-01 and subsection 16 of North Dakota Century Code section 43-15-01.
2. “”Pharmacy technician in training”” is a person who is enrolled in an academic experiential rotation program accredited by the american society of health systems pharmacists (ASHP)/accreditation council for pharmacy education (ACPE). A pharmacy technician in training, as they progress through their training program, may perform any of the duties of a registered pharmacy technician at the discretion of the pharmacist in charge and thepharmacist supervising their training program unless otherwise specified in the rules.
History: Effective October 1, 1993; amended effective July 1, 1996; January 1, 2024.
General Authority: NDCC 43-15-10(12)(14)(19)
Law Implemented: NDCC 43-15-10(12)(14)(19)” “61-02-07.1-03. Educational preparation.
1. To be eligible to be registered by the board of pharmacy as a pharmacy technician the person must have completed one of the following requirements:
a. Successful completion of an American society of health systems pharmacists accredited academic program;
b. An American society of health systems pharmacists accredited on-the-job training program.
2. Technician certification:
a. An applicant for registration as a pharmacy technician must have obtained certification by a national certification body approved by the board of pharmacy.
b. A technician registered after August 1, 1995, must obtain certification by a national certification body approved by the board of pharmacy.
c. The pharmacy technician certification board and national healthcareer association are approved certification bodies.
d. If a competency examination is developed by the national association of boards of pharmacy to foster transfer of registration between states, this will be accepted in lieu of certification.
History: Effective October 1, 1993; amended effective October 1, 2012; October 1, 2019.
General Authority: NDCC 28-32-02, 43-15-10(12)(14)(19)
Law Implemented: NDCC 43-15-10(12)(14)(19)” “61-02-07.1-07. Pharmacy technician registration requirements.
1. A pharmacy technician must register with the board of pharmacy on an annual basis.
2. The pharmacy technician will be assigned a registration number.
3. The board of pharmacy must provide the pharmacy technician with an annual registration card and pocket identification card.
4. The pharmacy technician certificate and annual registration card, or copy thereof, must be available or on file in the pharmacy where the pharmacy technician is employed.
5. The pharmacy technician must wear a name badge while in the pharmacy which clearly identifies the person as a “”pharmacy technician””.
6. Pharmacy technicians shall identify themselves as pharmacy technicians on all telephone conversations while on duty in the pharmacy.
7. The northland association of pharmacy technicians shall appoint annually three of their members as an advisory committee to the board of pharmacy.
8. Every registered pharmacy technician, within fifteen days after changing address or place of employment, shall notify the board of the change or make the necessary update on the board’s website. The board shall make the necessary changes in the board’s records.
9. A pharmacy technician having passed the reciprocity examination of the national association of boards of pharmacy, or any other examination approved by the board, shall be granted reciprocity and shall be entitled to registration as a registered pharmacy technician in North Dakota.
10. A pharmacy technician registered by the board may use the designations “”registered pharmacy technician”” and “”R. Ph. Tech.””.
11. A pharmacy technician holding a certificate of registration as a pharmacy technician in North Dakota may go on inactive status, and continue to hold a certificate of registration in North Dakota, provided that the technician on inactive status may not practice within North Dakota. A pharmacy technician on inactive status will not be required to meet the continuing education requirements of the board under chapter 61-02-07.1. In order for a pharmacy technician to change an inactive status registration to an active status of registration, the pharmacy technician must complete ten hours of approved pharmacy technician continuing education
and thereafter comply with the continuing education requirements of the board. Evidence of current certification by a national certification body approved by the board of pharmacy meets
this requirement.
12. In the case of loss or destruction of a certificate of registration, a duplicate can be obtained by forwarding the board an affidavit setting forth the facts.
13. Provisional registration for a member of the military or military spouse as defined in North Dakota Century Code section 43-51-01.
a. A provisional registration may be granted upon application for registration if the individual holds a registration or license as a pharmacy technician in another state and has worked under such license or registration for at least two of the last four years.
b. This provisional registration must be without fee until one year after the first renewal period has passed. This allows a maximum of two years without payment of a registration or renewal fee.
c. If the applicant does not meet all the criteria for registration under North Dakota laws or rules, the applicant must complete those qualifications before the applicant’s provisional registration period expires to continue registration.
History: Effective October 1, 1993; amended effective July 1, 1996; April 1, 2020; January 1, 2022; January 1, 2024.
General Authority: NDCC 43-15-10(12)(14)(19)
Law Implemented: NDCC 43-15-10(12)(14)(19), 43-51-11, 43-51-11.1″ “61-02-07.1-10. Pharmacy technician continuing education.
1. Each pharmacy technician shall complete at least ten hours of approved pharmacy technician continuing education every year as a condition of renewal of a registration as a pharmacy technician in North Dakota.
2. There may be no carryover or extension of continuing education units with the exception that continuing education units obtained twelve months prior to the beginning of each annual reporting period may be used in the current annual reporting period which begins March first of each year and ends the last day of February, or the previous reporting period. However, they may not be counted as credit in both reporting periods. The failure to obtain the required
ten hours of continuing education by the renewal date may result in a suspension for a minimum of thirty days, or a maximum of the period ending the date the continuing education is completed.
3. Pharmacy technicians shall maintain their own records. The records must be maintained for a two-year period.
4. The requirements of this section do not apply to a pharmacy technician applying for a first renewal of a registration.
5. A pharmacy technician registered with the board may make application to the board for a waiver of compliance with the pharmacy technician continuing education requirements and
may be granted an exemption by the board.
6. Upon request of the board, proof of compliance must be furnished to the board.
History: Effective July 1, 1996; amended effective January 1, 2005; January 1, 2010; October 1, 2019.
General Authority: NDCC 28-32-02, 43-15-10(12)(14)(19)
Law Implemented: NDCC 28-32-03” “61-02-07.1-11. Pharmacy technician in training.
A pharmacy technician in training must be designated as a pharmacy technician in training and will be allowed to practice the professional duties of a registered pharmacy technician as determined by the
pharmacist-in-charge and the supervising licensed pharmacist. Upon receipt of a request to have a person designated a pharmacy technician in training from a pharmacist-in-charge, the board, if
appropriate, shall register the person so enrolled as a pharmacy technician in training. The maximum amount of time to be registered as a technician in training is two years unless an extension is granted.
History: Effective July 1, 1996; amended effective January 1, 2005.
General Authority: NDCC 28-32-02, 43-15-10(12)(14)(19)
Law Implemented: NDCC 28-32-03” “61-02-07.1-12. Technicians checking technicians.
Activities allowed by law to be performed within a licensed pharmacy by a registered pharmacy technician in the preparation of a prescription or order for dispensing or administration may be
performed by one registered pharmacy technician, who may be a technician in training and verified by another registered pharmacy technician who may not be a technician in training, working in the same licensed pharmacy, under the following conditions:
1. The licensed pharmacy where the work is being conducted has policies and procedures specifically describing the scope of the activities to be verified through this practice, included in the policy and procedure manual required under section 61-02-01-18.
a. Training for the specific activity is reflected in a written policy.
b. A record of the individuals trained is maintained in the pharmacy for two years.
2. The pharmacy has a continuous quality improvement system in place to periodically verify the accuracy of the final product, including:
a. Recording any quality related events leading up to the final dispensing or administration of the drug prepared.
b. Recording any errors which actually reach the patient as a result of these activities.
c. Specific limits of acceptable quality related event levels before reassessment is required.
d. Consideration must be made for high-risk medications on the institute for safe medication practices (ISMP) list and specific monitoring, review, and quality assurance parameters must be instituted if any of these products are included in the pharmacy’s
technicians-checking-technicians program.
3. Any error must trigger pharmacist review of the process. This review and subsequent recommendations must be documented.
4. The pharmacy has a system in place to review all quality related events and errors recorded and takes corrective action based on the information to reduce quality related events and eliminate errors reaching the patient.
5. As always, the pharmacist-in-charge and the permitholder are jointly responsible for the final product dispensed or released for administration from the pharmacy.
History: Effective January 1, 2009; amended effective October 1, 2014; January 1, 2024.
General Authority: NDCC 43-15-10(12)(14)(19)
Law Implemented: NDCC 43-15-10(12)(14)(19)” “61-02-07.1-13. Pharmacy technician reinstatement.
If a registered pharmacy technician fails to pay the fee for a renewal registration within the time required, the executive director of the board shall cancel the registration for nonpayment. Upon application, the delinquent registrant may procure a renewed registration once the payment of all back registration fees, late fees, up to a maximum of five years and proof of ten hours of continuing
pharmaceutical education obtained within the past year are submitted, evidence of current certification by a national certification body approved by the board of pharmacy meets this requirement, provided there have been no disciplinary actions involved with the registration and the board is satisfied that the
applicant is a proper person to receive the same.
History: Effective January 1, 2011; amended effective January 1, 2024.
General Authority: NDCC 43-15-10(12)(14)(19)
Law Implemented: NDCC 43-15-10(12)(14)(19)
The data contained in this 2012 Annual Scorecard are accurate as of December 2012 . Because statutes and regulations are continually revised, the data are subject to change. These data have been verified with the state board of pharmacy. This scorecard is updated on an annual basis in order to incorporate statutory and regulatory changes. A new scorecard will be issued in July 2013.
Scoring rationale for Education and Training:
In order to protect the public and help ensure patient safety, it is important that pharmacy technicians are properly educated and trained. The most rigorous training is accredited training. The sole entity empowered to accredit pharmacy-technician training programs is the American Society of Health-System Pharmacists (ASHP). Please note that this is “programmatic accreditation” – not “institutional accreditation.” It is the content of the training program – as measured against established standards – that is being evaluated and accredited. Accredited training is vital to protecting patient safety because it means that a pharmacy-technician training program has met established quality standards to provide assurance and confidence to the public. For more information, please see http://www.ashp.org/menu/Accreditation/TechnicianAccreditation.aspx.
Scoring rationale for Certification:
Certification is the process by which a nongovernmental agency or association grants recognition to an individual who has met certain predetermined qualifications specified by that agency or association. This is often determined by an examination process. Numerous organizations have recommended that the certification exam conducted by the Pharmacy Technician Certification Board (PTCB) should be recognized as the sole, nationally-accredited certification exam for pharmacy technician certification – including the National Association of Boards of Pharmacy (NABP), the Texas State Board of Pharmacy (TSBP), and the U.S. Department of Veterans Affairs (VA). In a recent report, NABP recommended that states be encouraged to “recognize certification by the Pharmacy Technician Certification Board (PTCB).” Moreover, NABP performed a psychometric audit of the PTCB’s pharmacy technician certification examination (PTCE) in 2001 and determined that the PTCE is psychometrically sound, defensible, and valid. In May 2010, the TSBP awarded the PTCB with the Pharmacy Technician Certification Provider contract in Texas. PTCB was selected for the contract after a rigorous bidding and evaluation process that included formal reviews and evaluations from three independent psychometricians. TSBP confidently recognizes PTCB as the single provider of certification examinations for pharmacy technicians. In addition, in June 2010, the VA began requiring PTCB certification for VA pharmacy technicians employed at grade GS-6 and above.
Scoring rationale for Registration/Licensure:
Registration/licensure is the process by which the state maintains a list of all pharmacy technicians in the state and grants permission for an individual to work as a pharmacy technician in the state based on the applicant’s completion of all pre-requisites to registration/licensure – such as required training and certification.
Scoring rationale for Continuing Education:
Continuing education enables pharmacy technicians to fulfill their professional responsibility to maintain competence and up-to-date knowledge and skills in an environment of technological advances and increasingly complex, new medications and therapies.
Our Mission
The Emily Jerry Foundation is determined to help make our nation’s, world renowned, medical facilities safer for everyone, beginning with our babies and children. We are accomplishing this very important objective by focusing on increasing public awareness of key patient safety related issues and identifying technology and best practices that are proven to minimize the “human error” component of medicine. Through our ongoing efforts The Emily Jerry Foundation is working hard to save lives every day.
Recent Posts
Archives